Autor : Rey, DarĂo R.1
1Consultant physician in Pulmonology, Hospital Gral. de Agudos Dr. E. TornĂş
https://doi.org./10.56538/ramr.PHVI8364
Correspondencia : DarĂo R. Rey E-mail: darioraul.rey@gmail.com
Received: 12/9/2021
Accepted: 8/2/2022
The possibility exists that the global
workforce, which is approximately 140 million people, may suffer from an
occupational disease in practically every task it performs.1
However, despite that risk in the working population, few
published studies have estimated the incidence of these lesions or diseases,
their final cost in terms of medical expenses, the health of the affected indiÂvidual
and economic losses related to hours of work. Methodological failure,
inconsistency between the members of the research population or poor records
are factors that cause differences in the notification of occupational diseases
and probably contribute to this situation.2
As a consequence of changes in
manufacturing practices and the use of new materials, occupational medicine
specialists continue describing associations between new types of exposure and
chronic forms of diffuse parenchymal lung disease (DPLD). In order to
understand the association between expoÂsure and disease, specialized
physicians must see a high index of suspicion regarding the potential toxicity
of occupational and environmental exposure.
The diffuse interstitial lung
disease (DILD) related to the inadequate exÂposure or poor protection of
workers in different tasks, such as the usufruct of coal and asbestos deposits,
construction, manufacturing, work in shipyards and quarries, and agricultural
work, has been recognized as an occupational risk a long time ago.
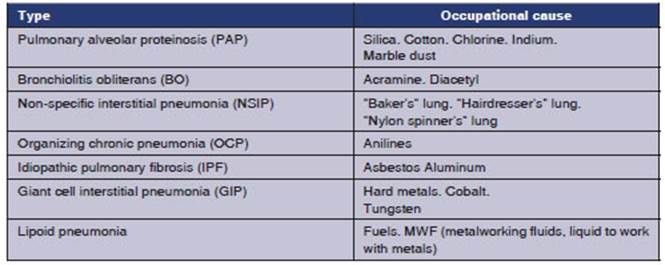
Interstitial lung diseases caused
by exposure to substances present in the workplace (OCCUP DILDs) are a group of
significant, preventable diseases. Many different agents cause
the OCCUP DILD, some of which are correctly defined, and some have been poorly
characterized. The list of causative agents keeps growing. Once considered as
“pneumoconiosis”, the list of known causes of OCCUP DILD extends far beyond
coal, silica and asbestos. Clinical, radiological and pathological
presentations of OCCUP DILD are similar to the non-work-related forms of the
disease due to the complex list of pulmonary depuration and repair of the lesion.
The attending physician must have a high index of suspicion and keep a detailed
occupational record in order to look for potential exposure whenever he/she
sees a patient with a DILD. Recognizing an OCCUP DILD is especially important
due to the implications related to primary and secondary prevention of diseases
among the exposed co-workers of the index case.
A study of the American Thoracic
Society pubÂlished in 2019 collected a literature review and data summary
regarding the occupational contriÂbution to the burden of the main
non-malignant respiratory diseases. The purpose of the study was mainly to
inform about diffuse interstitial fibrosis, hypersensitivity pneumonitis, sarcoidosis and other non-infectious, granulomatous
diseases.
The result indicated that
exposure in the workÂplace contributes essentially to the presence of multiple
chronic respiratory diseases, for example: asthma (16%), COPD (14%), chronic
bronchitis (13%), idiopathic pulmonary fibrosis (26%), hypersensitivity
pneumonitis (19%), granuloÂmatous diseases, including sarcoidosis (30%), pulmonary
alveolar proteinosis (29%) and
tuberculosis (2.3% in cases of silica exposure).
The conclusion of the study
establishes the urgent need to improve clinical suspicion and knowledge of the
public health regarding the contribution of occupational factors.3
Litow et al classify the OCCUP DILDs into four conditions (frequently
overlapped from the clinical point of view):
A. Pneumoconiosis,
defined as non-cancer pulÂmonary reaction to inhaled mineral or organic
dusts and resulting modification of the parenÂchyma structure.
B. Hypersensitivity
pneumonitis (HN), also known as “extrinsic allergic alveolitis”.
SubstanÂtial number of disorders of the organism’s immune response to the
inhalation of organic or chemiÂcal antigens, associated with histopathological, granulomatous-like changes.
C. Granulomatous diseases with
reaction of foreign body or chronic, immune diseases.
D. Interstitial diffuse
fibrosis, as a reaction to a severe pulmonary lesion, including the
inhalation of irritants.4
The following table shows some of
the numerÂous examples of OCCUP DILDs. Given the fact that a lot of cases in
the literature are referred to as exceptional, we should consider individual proÂpensity,
thus it may occur that one causative agent causes different pulmonary
interstitial responses.
The most widely known paradigms
of OCCUP DILDs are SILICOSIS and ASBESTOSIS. The acute form of silicosis may
adopt a pattern similar to pulmonary alveolar proteinosis
(PAP), whereas the non-tumor clinical form of asbestosis can be manifested as
an idiopathic pulmonary fibrosis (IPF).
OCCUP DILD SIMILAR TO PAP
PAP is an exceptional disease. Both
the surfactant secreted by the huge number of type II pneumoÂcytes
and the imperfect removal of the surfactant by alveolar macrophages play a role
in the physÂiopathology of this condition.
PAP is classified into two
essential forms: conÂgenital or acquired. The most common
variant is the acquired, and is divided into two sub-classes, autoimmune and
secondary, related to a causal factor. Acquired autoimmune PAP is the
most comÂmon. Secondary PAP is related to chronic inflamÂmatory processes,
tumors, hematologic diseases, immunosuppressor
syndromes, and inhalation of inorganic or organic particles.
Abraham and McEuen
studied twenty-four cases of PAP through optical and electron miÂcroscopy to
check if the PAP was associated with silica exposure. A large number of birefringent particles was found in 78% of the cases, as
opposed to control groups.
The environmental history that
was researched correlated well with the results of analyzed parÂticles, for
example, silica “sandblasting”, metal fumes in welders and cement particles.5
Silicosis is generally a chronic
pneumoconiosis. It is not common for the silicosis to adopt an acÂcelerated or
acute form; there aren’t many pubÂlications due to its low frequency of
presentation.
The accelerated variant occurs
between 5 to 10 years after exposure to dust with high concentraÂtions of
silica and if the person didn’t wear respiÂratory protection.
The acute variant is developed a
few years after very intense, brief exposure. It is similar to PAP, due to the
accumulation of lipoprotein PAS (periÂodic acid-Schiff) positive material in
the alveolar spaces; and the chest CT shows the radiographic crazy paving
pattern. It is difficult to differentiate silicoproteinosis
from PAP; the right thing to do is to have good occupational anamnesis. A
whole-lung lavage may delay the unfortunate evolution of this form of
silicosis.6-8
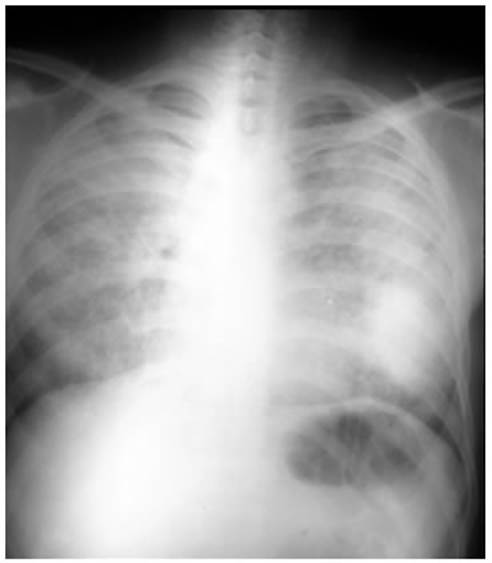
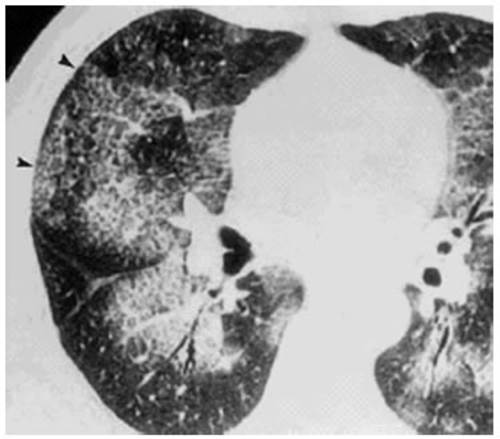
Attached is a case report of a
worker who perÂformed sandblasting of metal pieces for five years without
personal protection (he was unaware of the risk). Dyspnea FC IV and severe
hypoxemia were observed, and the chest CT showed the crazy paving pattern. The evolution
was fatal (Figures 1 and 2).
There are some publications in
the literature where the form of OCCUP DILD similar to PAP is induced by the
inhalation of indium (used in the electronic industry), aluminum (for the
industry of metallization, ship construction, used as dust or for welding),
cotton dust (it can be used in cotton bale carding machines), titanium (used by
paintÂers, mechanics, artificial jewelry manufacturers and titanium dioxide
producers); and there is a case report published in 2018 regarding years of
chronic inhalation of chlorine (used for tanning leather in a tannery).9-15
Recent epidemiological
research suggests that occupational and environmental exposure, as well as the
inhalation of irritants, contribute to cases of IPF, which then ceases to be
idiopathic.
Exposure to asbestos may create
radiographic and histopathological changes similar to
IPF.17
We should also include smoking, sawdust or wood dust, silica,
aluminum, and agricultural activities. The genetic variation among the
population may explain the differences in the susceptibility of the
presentation of varied patterns of interstitial disÂeases, even with the same
causative agent.
Between 1997 and 2000, Ekström et al invesÂtigated the effects of smoking in
occupational exÂposure and found that the fact of being a smoker represented a
great danger of developing severe forms of IPF, as well as a direct
relationship beÂtween the smoking load and higher risks.18
It is possible that in many IPF
patients, the fibrosis isn’t idiopathic because it was caused by occupational
exposure. Misdiagnosis can be due to inadequate anamnesis or lack of evident
suspicion in the causative agent. Some examples of the types of exposure that
contribute to the IPF pathogenia are: organic dust in
agriculture, livestock farming, metal, wood and asbestos dust.
One study conducted in Italy in
2008 found two groups of activities with a particularly high risk of developing
UIP that increased as long as the occupational exposure continued: gardeners,
veterinarians, farmers and metal workers. People who had their own experience
with exposure to the inhaled agent had a higher risk of developing UIP.19
Siderosis is the accumulation of excess iron oxÂide in the alveolar macrophages,
and was described in 1946 by Buckell et al.20
Gothi et al published a case report of
a welder with IPF associated with siderosis who had
been exposed for 40 years to metal fumes; and confirmed that it was originated
by siderosis through transbronchial
biopsy, with moderate functional loss one year after diagnosis, but without
major changes in the chest CT.21 AnÂother similar report is that
of McCormick et al, in which a patient showed IPF secondary to siderosis even though he had worn a welder’s protective
mask for 20 years.22
For a long time, it was thought
to be pneumoÂconiosis with “benign” evolution, given the lack of signs, symptoms
or fibrosis. It is also known as “arc-welders disease” because of the
aspiration of welding fumes with poor respiratory protection or without any
protection at all.23
Aluminum dust has also been an
IPF producer; there are publications about it in the literature. In 1990, Jederlinic et al published 9 cases of workers from a
factory of abrasives, with a mean exposure of 25 years. Radiographic and
functional findings were followed up with biopsy, confirming pulmoÂnary
aluminosis.24
In 2014, Raghu et al published a
similar case of IPF caused by aluminum with bad evolution in a worker who had
mechanized, sanded, drilled and rectified Corian for
16 years. This is a synthetic material consisting of one third of acrylic resin
and two thirds of aluminum trihydrate. Due to the
fact that it is thermoformable and acid-resistant, it
is used for the manufacture of kitchen and bathroom countertops and counters.25
Sawdust, in the form of inhalable
particles, can deposit on the pulmonary parenchyma and damage the workers’
health.
It is known that sawdust is
carcinogenic to huÂmans. Hancock et al carried out a meta-analysis of 85
studies in order to identify researches. The auÂthors showed an elevated risk
in workers exposed to sawdust, and a lower risk in those who worked with soft
wood. The study provided important certainty of this occupational cancer,
suggesting a different effect between hard and soft woods.26 The study of Gustafson et al
established that wood dust could contribute to the incidence of IPF. To that end,
they provided a 30-question questionÂnaire to 757 individuals with this
disease, which was answered by 181 of them. Through statistical studies they
found a higher risk among workers who had been exposed to the dust of hard
woods and birch wood.27
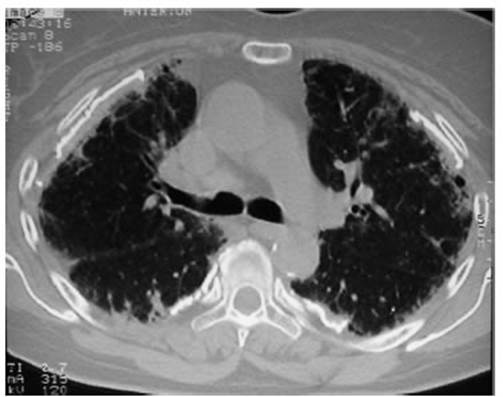
Attached is a case report of IPF
caused by asÂbestosis in an individual who worked as an electriÂcian and
manipulated insulation materials for 25 years without personal protection. The
chest CT shows pleural calcifications and peripheral signs of “honeycombing”
(Figure 3).
OCCUP DILD SIMILAR TO COMBINED PULMONARY FIBROSIS AND EMPHYSEMA (CPFE)
In 1990, Wiggins et al published
nine cases of CPFE, but the most important publication about this interstitial disease
was presented by Cottin et al with a cohort of 61
cases in which they emphaÂsized the predominance of the condition in men, its
relationship with smoking, acceptably preserved lung volumes and severely
decreased DLCO (difÂfusing capacity for carbon monoxide). Chest CT: fibrosis or
“honeycombing” in lower lung fields and emphysema in upper fields, with
extremely bad prognosis associated with pulmonary hyperÂtension. Survival is
lower in CPFE, compared to IPF or COPD.28,
29
Workers who manufacture tires may
suffer from pleural or pulmonary diseases because they are exposed to the
powder used to prevent adherence of the vulcanized surfaces of rubber. Vinaya et al published the case of a worker with CPFE who
had been exposed to powder aspiration for 26 years. The development of this
condition has also been associated with the use of agrochemicals30,
31 .
OCCUP DILD SIMILAR TO BRONCHIOLITIS OBLITERANS
Bronchiolitis obliterans
(BO) is an exceptional obÂstructive pulmonary disease of the small airways,
eventually fatal. It is characterized by the fibrosis of terminal and distal
bronchioles and an obstrucÂtive airflow pattern with progressive reduction of
the respiratory function. It is mostly seen as a non-infectious complication
and chronic rejection after lung transplantation. From the occupational point
of view, cases have been published of BO caused by artificial flavors of
popcorn, where diacetyl stands out. Other causes of
BO include exposure to toxins and inhaled gases, nitrogen oxides, sulfur
mustard, fiberglass.
American service members who
served in Iraq and Afghanistan suffered from BO after being exposed to a fire
in a sulfur mine, with elevated levels of SO2,
and possibly to emissions from open burn pits in military bases, where
batteries, plastic and waste had been disposed of by burning with jet fuel. Doujaiji and Al-Tawfiq, published
the case of a patient with BO caused by SO2
exposure in an oil refinery in the Persian Gulf.32,
33
In 1992 there was a series of 22
cases of BO among 257 textile workers in Spain who had been spraying the
fabrics with what seemed to be non-toxic dyes. Similar products caused a minor
situation in Algeria, with one death and 2 severe cases.34,
35
Experimental studies showed that
the textile paint that was used (Acramin FWR and Acramin FWN) was highly detrimental to the respiratory
system.36
Nanoparticles (NPs) are widely
used at presÂent, and, due to their size, they are included in the range of
breathable elements, because they remain in aerial suspension as aerosols. They
are used in the paper, pharmaceutical, paint, and cosÂmetic industries. Cheng
et al published the case of an individual who developed BO after exposure to
titanium NPs in paint. Recent experimental research revealed inflammatory
phenomena and formation of granulomas after unprotected expoÂsure to NPs.37
Cullinan et al published in 2012 six cases of BO in fiberglass workers, five of
which built vessels with that material, and the remaining worker was building a
cooling tower. The procedure was as follows: they had to build glass reinforced
plastic using resin mixed with styrene and phthalate plus methyl acetone
peroxide. The evolution was bad, with one death caused by respiratory failure,
two lung transplants and three survivors with poor lung function.38
On May 2000, the Bureau of
Occupational Health of Missouri, U.S.A, was informed about eight individuals
with BO who worked in a popÂcorn manufacturing plant. The national entity
(NIOSH, National Institute of Occupational Safety and Health) was notified of
this, and 135 workÂers were evaluated through questionnaires and spirometry. 117 workers finished the studies, and had a
respiratory obstruction rate 3.3 times higher than expected, and 2.6 times
higher than expected chronic cough rate.39 Diacetyl
is a volatile, water-soluble compound that vaporizes with heat, and is a
natural component of several foods, including beer and wine. Concentrated
formulae of diacetyl are used in the food industry,
as flavoring agents, and it is estimated that by 1995, 95 tons had been used
per year.40
If inhaled without respiratory protection or an adequate
ventilation, it can cause BO. Studies have been published in this regard.41
Diacetyl-induced BO among workers of the food industry is the most widely known
and published. After reviewing the literature, a publication has been found of
four cases in Brazil: young men, previously healthy, non-smokers. After 1 to 3
years of work without protection, there was obÂstructive spirometric
impairment between 25% and 44% of what was expected, and tomographic studies
showed air trapping, representative of BO, confirmed through biopsy.42
Just like Gulati and Kreiss, the BO diagnosis must include inspiratory and
expiratory chest CT, spirometry, and confirmaÂtory
biopsy can be avoided in case of compatible exposure. The identification of the
causative agent and its cessation is still fundamental for the index case, as
well as prevention among co-workers who participate in similar types of
exposure. Hygiene and safety include the monitoring of environmenÂtal
ventilation, the use of respiratory masks and covering food containers in order
to avoid leaks.43, 44
The notification, study and
follow-up of similar cases produced by diacetyl or
analogous substances in coffee processing plants have been reported by
Reid-Harvey and Bailey.45, 46
OCCUP DILD SIMILAR TO PLEUROPARENCHYMAL FIBROELASTOSIS (PPFE)
PPFE is an exceptional pulmonary
disease, with rare radiological and histopathological
clinical characteristics. Since the 2013 classification, it has been recognized
as an idiopathic interstitial pneumonia resulting from the combination of
visceral pleura fibrosis and fibroelastosis changes
at the subpleural pulmonary parenchyma. The chest CT
provides characteristic images that raise the suspicion of this disease.
Present as a cause of various diseases, there isn’t a unique triggering factor
for PPFE; reports have been published of chemotherapy sequelae,
bone marrow transplant, collagenopathy or as a
consequence of lung transÂplant rejection.47-52
Despite its uncommon incidence
and prevalence, there are some publications that relate the PPFE with exposure
to toxic substances in the workplace. In 2011, Piciucchi
et al published the case of an individual with PPFE who had high exposure to
asbestos; and in 2018, Xu et al published a similar
case report in which both asbestos and silica were associated with this
condition.53, 54
The publication of Okamoto et al
reports the case of a patient with PPFE who works as a dental technician. These
artisans are exposed to an unlimited number of materials such as silver,
cobalt, chrome, nickel, silica, indium and titanium. The prevalence of
pneumoconiosis among them is estimated at 4.5%-23.6%, with a mean exposure of
12.8 to 28.4 years.55-57
However, regarding this
particular disease, cases have been published caused by chronic inhalation of
aluminum in individuals without reÂspiratory protection, such as the reports of
Huang, Chino and Yabuchi. This metal generally proÂduces
OCCUP DILD similar to IPF, but the repair mechanisms of the pulmonary
parenchyma show individual sensitivity that causes this response.58-60
OCCUP DILD SIMILAR TO DESQUAMATIVE INTERSTITIAL PNEUMONIA (DIP)
First described by Averil Liebow et al, the main
characteristic of DIP is the accumulation of macroÂphages both in the lumen and
walls of the alveoli. Patients with this disease are usually heavy smokÂers;
and the masculine gender is predominant. Clinical symptoms are non-specific,
and due to the fact that patients generally respond to treatment with steroids,
this disease has a better prognosis than IPF.61
The literature has published case
reports about work-related DIP. In many cases, the patient is a heavy smoker.
Abraham and Hertzberg studied 62
samples of DIP confirmed by biopsy using electronic microsÂcopy and X-ray
scattering analysis, in search of inÂorganic particles. Seven out of seventeen
analyzed cases had an occupational history of exposure to that type of
elements, and high concentrations of those were found in their biopsies. The
authors certified specific classes of particles in 92% of the patients, that is
why they suggested a relationship between DIP and the worker’s profession.62
Interstitial lung diseases are
not commonly associated with primary aluminum production. OCCUP DILD induced by
aluminum is very rare, but the experience suggests that exposure to metal fumes
and dust may cause diffuse changes in the parenchyma, such as granulomas, PAP
or DIP.63
Lijima et al published the case report of a worker with a smoking load of 60
packs per year who developed DIP caused by aluminum exposure and showed
excellent evolution after corticosteroid treatment and smoking cessation. The
oriented anamnesis revealed certain tasks of aluminum processing that include
melting the metal in molds for engine covers and polishing the pieces.64
Blin et al published the case report of a patient with DIP who had been
exposed to asbestos workÂing as a plumber and then worked as a caster, with
potential exposure to copper, bronze, iron, aluminum and zirconium alloys. He
had a smokÂing history of 30 packs per year, and had quitted 10 years before
the consultation.65
Zirconium is a
corrosion-resistant material used in the aerospace, airline, and nuclear power
industries. Thanks to scientific research tests, it is known that after
repeated use, it may cause a hypersensitivity skin reaction, forming granuÂlomas
of epitheliolid cells. Experimental studies showed
lung alterations such as granulomas and interstitial fibrosis after exposure to
zirconium. There are few publications in humans regarding pulmonary diseases
related to zirconium.66
Kawabata et al studied 31 patients
with DIP of varied etiologies, where 93% of the patients were male smokers.
These patients had been followed-up for more than 99 months. 14 patients who
had been monitored for a longer period showed 5 cases of IPF and 4 of lung
cancer. The conclusion of this study was that, with time, DIP can progress to
IPF, despite the treatment.67
Coal power plants are still an
important source of power supply worldwide, so miners will continue suffering
from pneumoconiosis and dying because of it. After the approval of the Federal
Coal Mine Health and Safety Act of 1969 in the U.S.A., measures were adopted to
restrict exposure, thus causing a persisting reduction in the prevalence of
pneumoconiosis among coal workers in the U.S.A. from 1970 to 2000.68
Jelic et al studied, in 25 autopsies with microsÂcopy and polarized light,
the number of intramacÂrophageal silica particles and
the degree of fibrosis in antracosilicosis, and in 21
cases, respiratory bronchiolitis associated with smoking. The proporÂtion of
particles found was significantly higher in the cases of occupational disease
(331:4 p < 0.001).
The presence of intra-alveolar
macrophages full of particles of different fibrosis levels indicated a new
disease: chronic DIP, as a predecessor of difÂfuse fibrosis and emphysema
related to stone coal. Among smoking miners, smoking-related fibrosis wasn’t
significant in relation to occupational DIP.
Counting the number of
particle-laden macÂrophages in the BAL (bronchoalveolar
lavage) of miners can predict the disease and suggest prevenÂtion measures.69
OCCUP DILD SIMILAR TO LIPOID PNEUMONIA (LP)
LP, caused by the presence of
lipids in the alveoli, is a rare condition. It is classified into two main groups,
depending on the source of the oily subÂstance: exogenous or endogenous. It has
an insidiÂous onset and non-specific respiratory symptoms, such as dyspnea,
fever or cough. Tomographic findings include nodules, ground glass, crazy pavÂing
or consolidation. Since this condition is not suspected, the diagnosis is
delayed or unnoticed. It may simulate many other lung diseases, includÂing
carcinoma. LP is a chronic foreign body reacÂtion, identified with lipid-laden
macrophages. The diagnosis requires a high index of suspicion and may be
confirmed through respiratory samples showing the macrophages. Treatment
guidelines are not properly defined. The aim of the diagnosis in the exogenous
form of the disease is to avoid exposure. Steroids as a therapeutic option seem
promising.70
Normally, exogenous LP is
associated with the accidental inhalation of oil laxatives to treat conÂstipation,
as can be seen in the series of 44 cases of Gondouin
et al, where only 4 patients had an occupational origin.71 Fire-eaters are a workgroup
inside the entertainment industry. Possible comÂplications of their work must
be taken into considÂeration. The normal procedure consists in blowing the pyrofluid against a burning stick, so there’s the risk that
the fire may propagate to the mouth.72
The first description in the
literature about this occupational hazard was related to accidental aspiration
and was reported in 1971. Since then, there were other publications of LP in
fire-eaters. It is easier to make the diagnosis of the clinical condition after
a good patient inquiry.73-76
According to Gentina
et al, fire-eaters use difÂferent pyrofluids, the
most common being the Kerdan, an oil product of
reduced viscosity that unfortunately spreads rapidly through the bronÂchial
tree after accidental inhalation.
Between October 1996 and January
2001, these authors reviewed seventeen subjects, ten of which had been working
as fire-eaters for years. Their mean age was 24.5 years, and they showed
pleural pain (100%), dyspnea (93.3%), fever of more than 38.5°C (93.3%), cough
(66.6%) and hemoptysis (26.6%). One patient suffered an hemodynamic shock and
had to be treated at the ICU, where he/ she developed a pleuropulmonary
infection. Upon discharge, he/she had a good evolution but with sequelae (opacity in the middle lobe and pleural scar).
Complete pulmonary resolution occurred after 14 days in fifteen patients.77
Apparently, according to
consulted publicaÂtions, it is very common for the employees of gas stations to
practice fuel siphonage, which may be a potential
risk of aspirating liquids or vapor. Directed inquiry allows us to suspect the
cause of this particular LP and obtain diagnosis and treatÂment success.78-82
Sometimes, suspicion requires a
thorough inÂquiry. The patient from the study of Dhouib
et al lubricated cars for eight years with an oil spray without any
respiratory protection; this delayed his/her etiologic diagnosis for 2 years.83
Han et al reported three cases of
LP caused by the use of a paraffin aerosol. Their diagnosis was confirmed
through biopsy and through the study of the samples by x-ray diffraction. The
workers had long-term occupational exposure to paraffin, and the environmental
concentration of the product in the workplace was specifically higher than the
levels measured outdoors.84 Very exceptional cases of LP
were caused by herbicides or solvents used for dry cleaning in the laundry.85, 86
In short, a good patient
occupational inquiry is extremely useful because it generally provides us the
key to guide and elucidate the diagnosis.
REFERENCES
1. Blanc PD, Iribarren
C, Trupin L, et al. Occupational expoÂsures and the
risk of COPD: dusty trades revisited. Thorax. 2009;64:6-12.
https://doi.org/10.1136/thx.2008.099390
2. Schulte PA. Characterizing the
burden of occupational injury and disease. J Occup
Environ Med. 2005;47:607-22. https://doi.org/10.1097/01.jom.0000165086.25595.9d
3. Blanc P, Annesi-Maesano
I, Balmes J, et al.- The OccuÂpational Burden of
Nonmalignant Respiratory Diseases An Official American Thoracic Society and
European ReÂspiratory Society Statement. Am J Resp Crit Care Med. 2019;199:1312-35.
https://doi.org/10.1164/rccm.201904- 0717ST
4. Litow
F, Petson E, Bohnker B, et
al. Occupational InterstiÂtial Lung Diseases J Occup
Environ Med. 2015; 57: 1250-5. https://doi.org/10.1097/JOM.0000000000000608
5. Abraham J, McEuen
D. Inorganic particulates associated with pulmonary alveolar proteinosis: SEM and X-ray miÂcroanalysis results. Appl Pathol. 1986;4:138-46.
6. Nakládalová
M, Štěpánek L, Kolek
V, et all. A case of accelÂerated silicosis Occup Med
(Lond). 2018;68:482-4.
https://doi.org/10.1093/occmed/kqy106
7. Hutyrová
B, Smolková P, Nakládalová
M, et al. Case of acÂcelerated silicosis in a sandblaster Ind
Health. 2015;53:178- 83. https://doi.org/10.2486/indhealth.2013-0032
8. Stafford M, Cappa A, Weyant M, et al.Treatment of Acute Silicoproteinosis
by Whole-Lung Lavage. Semin Cardiothorac
Vasc Anesth. 2013;17:152-9.
https://doi.org/10.1177/1089253213486524
9. Cummings K, Nakano M, Omae K, et al. Indium Lung Disease. Chest 2012;141:1512-21.
https://doi.org/10.1378/chest.11-1880
10. Cummings K, Donat W, Ettensohn D, et al.
Pulmonary Alveolar Proteinosis in Workers at an
Indium Processing Facility. Am J Respir Crit Care Med.2010;181:458-64.
https://doi.org/10.1164/rccm.200907-1022CR
11. Omae K, Nakano
M, Tanaka A, et al. Indium lung--case reports and epidemiology. Int
Arch Occup Environ Health. 2011;84:471-7.
https://doi.org/10.1007/s00420-010-0575-6
12. Keller C. Pulmonary alveolar proteinosis in a painter with elevated pulmonary
concentrations of titanium. Chest. 1995;108:277-80.
https://doi.org/10.1378/chest.108.1.277
13. Thind
G. Acute pulmonary alveolar proteinosis due to exÂposure
to cotton dust. Lung India. 2009;26:152-4. https://doi.org/10.4103/0970-2113.56355
14. Miller R, Churg
A, Hutcheon M, et al. Pulmonary AlÂveolar Proteinosis
and Aluminum Dust Exposure. Am Rev Respir Dis
1984;130:312-5. https://doi.org/10.1164/arrd.1984.130.2.312
15. Rey D, Gonzalez J. Pulmonary
alveolar proteinosis secondÂary to chronic chlorine
occupational inhalation. J Lung Pulm Respir Res. 2018;5:100-3.
https://doi.org/10.15406/jlprr.2018.05.00171
16. Travis W, Costabel
U, Hansell, et al. An official American Thoracic
Society/European Respiratory Society Statement: Update of the International
Multidisciplinary Classification of the Idiopathic Interstitial Pneumonias. Am
J Respir Crit Care Med
2013;188:733-48. https://doi.org/10.1164/rccm.201308-1483ST
17. Stufano
A, Scardapane A, Foschino
M, et al. Clinical and radiological criteria for the differential diagnosis
between asbestosis and idiopathic pulmonary fibrosis: Application in two cases.
Med Lav. 2020;112:115-22.
18. Ekström
M, Gustafson T, Boman K, et al. Effects of smokÂing,
gender and occupational exposure on the risk of severe pulmonary fibrosis: a
population-based case-control study. BMJ Open 2014;4:e004018.
https://doi.org/10.1136/bmjoÂpen-2013-004018
19. Paolocci
G, Folletti I, Torén
K, et al. Occupational risk facÂtors for idiopathic pulmonary fibrosis in
Southern Europe: a case-control study BMC Pulm Med
2018;18:75. https://doi.org/10.1186/s12890-018-0644-2
20. Buckell M, Garrad J, Jupe
M, et al. The incidence of sideroÂsis
in iron turners and grinders. Br J Ind Med
1946;3:78-82. https://doi.org/10.1136/oem.3.2.78
21. Gothi
D, Satija B, Kumar S, et al. Interstitial Lung
Disease due to Siderosis in a Lathe Machine Worker.
Indian J Chest Dis Allied Sci 2015;57:35-7.
22. McCormick L, Goddard M, Mahadeva R. Pulmonary fibrosis secondary to siderosis causing symptomatic respiratory disease: a case
report. Journal of Med Case Rep 2008; 2:257.
https://doi.org/10.1186/1752-1947-2-257
23. Doig
A, McLaughlin A. X-ray appearances of the lungs of electric arc welders. Lancet
1936;1:771-5. https://doi.org/10.1016/S0140-6736(00)56868-8
24. Jederlinic
P, Abraham J, Himmelstein J, et al. Pulmonary
fibrosis in aluminum oxide workers. Investigation of nine workers, with
pathologic examination and microanalysis in three of them Am Rev Respir Dis 1990; 142:1179-84.
https://doi.org/10.1164/ajrccm/142.5.1179
25. Raghu G, Xia D, Schmidt R, et
al. Pulmonary Fibrosis Associated with Aluminum Trihydrate
(Corian) Dust) N Engl J Med
2014;370: 2154-6. https://doi.org/10.1056/NEJMc1404786
26. Hancock D, Langley M, Chia K,
et al. Wood dust exÂposure and lung cancer risk: a meta-analysis Occup Environ Med 2015;72:889-98.
https://doi.org/10.1136/oemed-2014-102722
27. Gustafson T, Dahlman-Höglund A, Nilsson K, et al. Occupational
exposure and severe pulmonary fibrosis. Respir Med 2007; 101:2207-12.
https://doi.org/10.1016/j.rmed.2007.02.027
28. Wiggins J, Strickland B,
Turner-Warwick M. Combined cryptogenic fibrosing alveolitis and emphysema: the value of high resolution
computed tomography in assessment. Respir Med.
1990;84:365-9. https://doi.org/10.1016/S0954-6111(08)80070-4
29. Cottin
V, Nunes H, Brillet PY, et
al. Combined pulmonary fibrosis and emphysema: A distinct under-recognized
entity. Eur Respir J.
2005;26:586-93. https://doi.org/10.1183/09031936.05.00021005
30. Karkhanis
VS, Joshi JM. Combined pulmonary fibrosis and emphysema in a tyre industry worker. Lung India. 2012;29:273-6.
https://doi.org/10.4103/0970-2113.99116
31. Daniil
Z, Koutsokera A, Gourgoulianis
K. Combined pulÂmonary fibrosis and emphysema in patients exposed to
agrochemical compounds: Correspondence. Eur Respir. J 2006;27:434-39.
https://doi.org/10.1183/09031936.06.0012 4505
32. King MS, Eisenberg R, Newman JH,
et al. Constrictive bronchiolitis in soldiers returning from Iraq and AfÂghanistan.
N Engl J Med. 2011; 365:222-30. https://doi.org/10.1056/NEJMoa1101388
33. Doujaiji B, Al-Tawfiq JA. Hydrogen
sulfide exposure in an adult male. Ann Saudi Med. 2010;30:76-8.
https://doi.org/10.5144/0256-4947.59379
34. Moya
C, Antó J, Newman-Taylor A. Outbreak of orÂganising pneumonia in textile printing sprayers. LanÂcet.
1994;344:498-502. https://doi.org/10.1016/S0140-6736(94)91896-1
35. Ould
Kadi F, Mohammed-Brahim B, Fyad A, et al. Outbreak of pulmonary disease in textile dye
sprayers in Algeria. Lancet 1994;344:962-3.
https://doi.org/10.1016/S0140-6736(94)92323-X
36. Clottens
F; Verbeken E; Demedts M,
et al. Pulmonary toxÂicity of components of textile paint linked to the Ardystil syndrome: intratracheal
administration in hamsters OcÂcup Environ Med
1997;54:376-87. https://doi.org/10.1136/oem.54.6.376
37. Cheng TH, Ko
FC, Chang JL, et al. Bronchiolitis Obliterans
Organizing Pneumonia Due to Titanium Nanoparticles in Paint. Ann Thorac Surg. 2012;93:666-9.
https://doi.org/10.1016/j.athoracsur.2011.07.062
38. Cullinan
P, McGavin CR, Kreiss K, et
al. Obliterative bronchiolitis in fibreglass
workers: a new occupational disease? Occup Environ Med. 2013; 70:357-.9. https://doi.org/10.1136/oemed-2012-101060
39. Kreiss
K, Gomaa A, Kullman G, et
al. Clinical bronchiolÂitis obliterans in workers at
a microwave-popcorn plant. N Engl J Med.
2002;347:330-8. https://doi.org/10.1056/NEJMoa020300
40. Harber
P, Saechao K, Boomus C. Diacetyl-induced lung disease. Toxicol
Rev. 2006;25:261-72. https://doi.org/10.2165/00139709-200625040-00006
41. Palmer S; Flake G, Kelly F,
et al. Severe airway epiÂthelial injury, aberrant repair and bronchiolitis oblitÂerans develops after diacetyl
instillation in rats. PLoSOne. 2011;6(3):e17644.
https://doi.org/10.1371/journal.pone.0017644
42. Cavalcanti Zdo R, Albuquerque Filho AP,
Pereira CA, et al. Bronquiolite associada
à exposição a aroma artificial
de manteiga em trabalhadores de uma
fábrica de biscoiÂtos no Brasil J Bras Pneumol. 2012;38:395-9. https://doi.org/10.1590/S1806-37132012000300016
43. Gulati
M,Teng A. Small Airways Disease Related to OcÂcupational
Exposures. Clin Pulm Med
2015;22:133-40. https://doi.org/10.1097/CPM.0000000000000094
44. Kreiss
K. Occupational causes of constrictive bronchiolitis Curr
Opin Allergy Clin Immunol. 2013;13:167-172. https://
doi.org/10.1097/ACI.0b013e32835e0282
45. Reid-Harvey R, Fechter-Leggett E, Bailey R, et al. The burden of
respiratory abnormalities among workers at cofÂfee roasting and packaging
facilities. FrontPublic Health. 2020; 8:5.
https://doi.org/10.3389/fpubh.2020.00005
46. Bailey R, Cox-Ganser J, Duling M, et al.
Respiratory Morbidity in a Coffee Processing Workplace with Sentinel Obliterative Bronchiolitis Cases. Am J Ind Med. 2015;
58(12):1235-45. https://doi.org/10.1002/ajim.22533
47. Travis W, Costabel
U, Hansell D, et al. ATS/ERS ComÂmittee on Idiopathic
Interstitial Pneumonias. An official American Thoracic Society/European
Respiratory Society statement: update of the international multidisciplinary classificationof the idiopathic interstitial pneumonias. Am
J Respir Crit Care Med.
2013;88:733-48. https://doi.org/10.1164/rccm.201308-1483ST
48. von der Thüsen
J, Hansell D, Tominaga M,
et al. PleuroÂparenchymal fibroelastosis
in patients with pulmonary disease secondary to bone marrow transplantation.
Mod Pathol. 2011;24:1633-9.
https://doi.org/10.1038/modÂpathol.2011.114
49. Beynat-Mouterde
C, Beltramo G, Lezmi G, et
al. PleuÂroparenchymal fibroelastosis
as a late complication of chemotherapy agents. Eur Respir J. 2014;44:523-7.
https://doi.org/10.1183/09031936.00214713
50. Enomoto Y, Nakamura Y, Colby T, et al. Radiologic pleuropaÂrenchymal fibroelastosis-like lesion in connective tissue disÂease-related
interstitial lung disease PLoS ONE. 2017;12:
e0180283. https://doi.org/10.1371/journal.pone.0180283 J
https://doi.org/10.1371/journal.pone.0180283
51. Jacob J, Odink A, Brun AL, et al. Functional
associations of pleuroparenchymal fibroelastosis
and emphysema with hypersensitivity pneumonitis. Respir Med. 2018;138: 95- 101.
https://doi.org/10.1016/j.rmed.2018.03.031
52. Hirota T, Fujita M, Matsumoto T, et al. PleuroparenÂchymal fibroelastosis as a manifestation of chronic
lung rejection? Eur Respir
J. 2013;41:243-5. https://doi.org/10.1183/09031936.00103912
53. Piciucchi
S; Tomassetti S:; Cason G y col. High resolution CT
and histological findings in idiopathic pleuroparenÂchymal
fibroelastosis: Features and differential diagnosis. Respir Res 2011;12:111.
https://doi.org/10.1186/1465-9921-12-111
54. Xu
L, Rassaei N, Caruso C. Pleuroparenchymal
fibroÂelastosis with long history of asbestos and
silicon exÂposure. Int J Surg
Pathol. 2018;26:190-3.
https://doi.org/10.1177/1066896917739399
55. Okamoto M, Tominaga M, Shimizu S, et al. Dental TechniÂcians'
Pneumoconiosis Intern Med. 2017;56:3323-6. https://doi.org/10.2169/internalmedicine.8860-17
56. Kahraman
H, Koksal N, Cinkara M, et
al. PneumoconioÂsis in dental technicians: HRCT and pulmonary function
findings. Occup Med (Lond).
2014; 64:442-7. https://doi.org/10.1093/occmed/kqu047
57. Ergün
D, Ergün R, Ozdemir C,
et al. Pneumoconiosis and respiratory problems in dental laboratory
technicians: analysis of 893 dental technicians. Int
J Occup Med Environ Health. 2014; 27:785-96.
https://doi.org/10.2478/s13382-014-0301-9
58. Huang Z, Li S, Zhu Y, et al. PleuroparenchymalfibroelasÂtosis
associated with aluminosilicate dust: a case report Int J Clin Exp
Pathol. 2015;8:8676-9.
59. Chino H; Hagiwara E; Midori S
y col. Pulmonary AlumiÂnosis Diagnosed with In-air Microparticle Induced X-ray Emission Analysis of Particles
(Intern Med 2015;54:2035- 40. https://doi.org/10.2169/internalmedicine.54.4246
60. Yabuuchi Y, Goto H, Nonaka M, et al. A case of airway aluminosis with likely
secondary pleuroparenchymal fibroelastosis.
Multidiscip Resp Med 2019; 14:15. https://doi.org/10.1186/s40248-019-0177-4
61. Liebow
A, Steer A, Billingsley J. Desquamative interstitial
pneumonia. Am. J. Med. 1965;39:369-404.
https://doi.org/10.1016/0002-9343(65)90206-8
62. Abraham J, Hertzberg M.
Inorganic particulates asÂsociated with desquamative
interstitial pneumonia. Chest.1981; 80:67e70.
https://doi.org/10.1378/chest.80.1_ Supplement.67S
63. Taiwo
O. Diffuse Parenchymal Diseases Associated With Aluminum Use and Primary
Aluminum Production. J OcÂcup Environ Med. 2014
May;56(5 Suppl):S71-2.
https://doi.org/10.1097/JOM.0000000000000054
64. Iijima Y, Bando M, Yamasawa H, et al. A case of mixed dust pneumoconiosis with desquamative
interstitial pneumonia-like reaction in an aluminum welder. Resp
Case Rep 2017; 20:150-3, https://doi.org/10.1016/j.rmcr.2017.02.002
65. Blin
T, De Muret A, Teulier M,
et al. Desquamative inÂterstitial pneumonia induced
by metal exposure. A case report and literature review Sarcoidosis.
Vasc Diffuse Lung Dis.2020;37:79-84.
66. Werfel U, Schneider J, Rödelsperger K, et al. Sarcoid
granuÂlomatosis after zirconium exposure with
multiple organ involvement Eur Respir
J. 1998; 12:750. https://doi.org/10.1183/09031936.98.12030750
67. Kawabata Y, Takemura T, Hebisawa A, et al. Desquamative interstitial pneumonia may progress to lung fibrosis as characterized radiologically. Respirology
2012;17:1214-21. https://doi.org/10.1111/j.1440-1843.2012.02226.x
68. Cohen R, Petsonk
E, Rose C, et al. Lung Pathology in U.S. Coal Workers with Rapidly Progressive
Pneumoconiosis Implicates Silica and Silicates. Am J Respir
Crit Care Med 2016;193:673-80.
https://doi.org/10.1164/rccm.201505-1014OC
69. Jelic T, Estalilla O, Sawyer-Kaplan P, et
al. Coal mine dust desquamative
chronic Interstitial pneumonia: A precursor of dust-related diffuse fibrosis
and of emphysema. Int J OcÂcup
Environ Med 2017;8:153-65. https://doi.org/10.15171/ijoem.2017.1066
70. Hadda
V, Khilnani G. Lipoid pneumonia: an overview Expert
Rev. Resp. Med. 2010;4:799-807. https://doi.org/10.1586/ers.10.74
71. Gondouin
A, Manzoni P; Ranfaing E, et al. Exogenous lipid
pneumonia: a retrospective multicentre study of 44
cases in France. Eur Respir J. 1996; 9 :1463-9.
https://doi.org/10.1183/09031936.96.09071463
72. Bankier
A, Brunner C, Friedrich L, Lomoschitz F, Mallek R, Reinhold M. Pyrofluid
inhalation in "Fire-eaters": SeÂquential findings on CT. J Thorac Imaging. 11999;14:303-6. https://doi.org/10.1097/00005382-199910000-00012
73. Gerbeaux
J, Couvreur J, Tournier G,
et al. Une cause rare de pneumatocele
chez l'enfant: L'ingestion d'hydrocarbures (kerdane). Ann
Med Interne (Paris). 1971;122: 589-96.
74. Beermann
B, Christensson T, Moller P, et al. Lipoid pneumoÂnia:
an occupational hazard of fire eaters Brit Med J. 1984; 289:1728-9.
https://doi.org/10.1136/bmj.289.6460.1728
75. Şahin
F, Yıldız P. Fire Eater's Pneumonia: One of
the Rare Differential Diagnoses of Pulmonary Mass Images. Iran J Radiol. 2011; 8:50-2.
76. Behnke
N, Breitkreuz E, Buck C, et all. Acute respiratory
distress syndrome after aspiration of lamp oil in a fire-eater: a case report. JMed Case Rep. 2016; 10:193.
https://doi.org/10.1186/s13256-016-0960-1
77. Gentina
T, Tillie-Leblond I, Birolleau
S, et al. Fire-Eater's Lung Seventeen Cases and a Review of the Literature. MedÂicine.
2001; 80:291-97. https://doi.org/10.1097/00005792-200109000-00002
78. Gowrinath
V, Shanthi V, Sujatha G, et
al. Pneumonitis folÂlowing diesel fuel siphonage. Resp Med Case Rep. 2012;5:9- 11.
https://doi.org/10.1016/j.rmedc.2011.11.010
79. Prasad KT, Dhooria S, Bal A, Agarwal R, Sehgal IS. An Unusual
Cause of Organizing Pneumonia: Hydrocarbon Pneumonitis. J Clin
Diagn Res. 2017;11(6):OD03-OD04.
https://doi.org/10.7860/JCDR/2017/24194.10008
80. Yampara Guarachi
GI, Barbosa Moreira V, Santos FerÂreira A, Sias SM, Rodrigues CC, Teixeira GH. Lipoid pneumonia in a gas station attendant. Case Rep Pulmonol. 2014;2014:358761. https://doi.org/10.1155/2014/358761
81. Venkatnarayan
K, Madan K, Walia R, Kumar
J, Jain D, Guleria R. "Diesel siphoner's lung": Exogenous lipoid pneumonia following
hydrocarbon aspiration. Lung India. 2014;31(1):63-6.
https://doi.org/10.4103/0970-2113.125986
82. Shrestha
TM, Bhatta S, Balayar R, Pokhrel S, Pant P, Nepal G. Diesel siphoner's
lung: An unusual cause of hydrocarbon pneumonitis. Clin
Case Rep. 2020;9(1):416-9. https://doi.org/10.1002/ccr3.3545
83. Dhouib
F, Hajjaji M, Jmal Hammami K, et al. Occupational Exogenous Lipoid Pneumonia:
A Commonly Delayed DiagÂnosis. Respir Case Rep. 2019;8: 6-9.
https://doi.org/10.5505/respircase.2019.19971
84. Han C, Liu L, Du S, et al. Investigation
of rare chronic lipoid pneumonia associated with occupational exposure to
paraffin aerosol. J Occup Health. 2016;58:482-8.
https://doi.org/10.1539/joh.16-0096-CS
85. Hotta
T, Tsubata Y, Okimoto T, et
al. Exogenous lipoid pneumonia caused by herbicide inhalation. Respir Case Rep.
2016;4 e00172. https://doi.org/10.1002/rcr2.172
86. Park S, Park J, Lee J. A case
of lipoid pneumonia associated with occupational exposure to solvents in a
dry-cleaning worker. RespirCase Rep. 2021;,9 e00762. https://doi.org/10.1002/rcr2.762