Autor : De Vito, Eduardo L.1-2
1Instituto de Investigaciones MÃĐdicas Alfredo Lanari, Faculty of Medicine, University of Buenos Aires. 2Centro del Parque, Respiratory Care, Buenos Aires, Argentina.
https://doi.org./10.56538/ramr.UUFV3942
Correspondencia :Eduardo L. De Vito. E-mail: eldevito@gmail.com
ABSTRACT
This article analyzes certain
evolutionary aspects of gas exchange, lung development, the respiratory pump,
the acid-base status and control of ventilation in relation to a significant
event: the passing from aquatic to terrestrial life. By studying this, we can
understand certain aspects that are present in the clinical practice: Why do
people with extreme respiratory muscle weakness breathe as frogs? (frog breathing); why do newborns with breathing difficulties
have nasal flaring and expiratory grunting?; how is it possible that abdominal
muscles, which are typically expiratory, assist with inspiraÂtion in cases of
diaphragmatic paralysis?; why does the breathing pattern of respiratory failure
has less variability and becomes more rigid? and, finally,
is it possible to imagine a neutral pH that doesnât have the 7.0 value?; whatâs
the use of this knowledge, and how should gases in hypothermia be interpreted?
Water-to-land transition is one
of the most important and inspiring major transitions of vertebrate evolution.
Given the amazing diversity of living organisms, it is tempting to imagine an
enormous amount of evolutionary adaptation processes to solve the different
challenges of living on earth faced by each species. There are certain early development
processes that share some crucial factors, and some of the close and distant
gene regulatory networks are conserved. We are witnesses of clinical findings
that serve as testimony of the species that lived in remote times and left us
their evoÂlutionary history.
Key words: Acid-base equilibrium, Hypothermia, Imidazole, Biological evolution,
Respiratory paralysis, Respiratory center
RESUMEN
Este artículo analiza ciertos aspectos evolutivos
en el intercambio gaseoso, el desaÂrrollo pulmonar, la bomba respiratoria, el
estado ácido-base y el control de la ventilaÂción en
relación con un evento trascendente: el pasaje de la vida
acuática a la terresÂtre. Su estudio puede permitir comprender ciertos
aspectos con los que lidiamos en la práctica clínica: ÂŋPor
qué las personas con debilidad muscular respiratoria extrema respiran
como ranas (respiración frog)?, ÂŋPor
qué los recién nacidos con dificultad respiratoria tienen aleteo
nasal y quejido espiratorio?, Âŋcómo es posible que los músÂculos
abdominales, típicamente espiratorios, asistan a la inspiración
en casos de la parálisis diafragmática?, Âŋpor qué en la
insuficiencia respiratoria el patrón respiratorio tiene menos
variabilidad y se torna más rígido? y, por último, Âŋes
posible imaginar un pH neutro que no tenga el valor de 7,0, para qué
sirve este conocimiento y como se deben interpretar los gases en hipotermia?
La transición del agua a la tierra es una de las
más importantes e inspiradoras de las grandes transiciones en la
evolución de los vertebrados. Ante la sorprendente diversiÂdad de
organismos vivos, es tentador imaginar una cantidad enorme de adaptaciones
evolutivas para resolver los diferentes desafíos que cada especie tiene
para la vida en la tierra. Hay desarrollos tempranos que comparten algunos
factores cruciales y algunas de las redes genéticas regulatorias
cercanas y lejanas están conservadas. Somos testigos de hallazgos
clínicos que son el testimonio de especies que han vivido en
épocas remotas y nos han legado su historia evolutiva.
Palabras clave: Equilibrio ácido-base, Hipotermia, Imidazol,
Evolución biológica, Parálisis respiratoria, Centro
respiratorio
Received: 6/20/2022
Accepted: 8/9/2022
Plus ça change, plus câest la même chose.
Alphonse Karr (1808-1890)
The objective of this article is
to analyze certain breathing-related evolutionary aspects, particuÂlarly gas
exchange, lung development, the respiÂratory pump, the acid-base status and
control of ventilation in relation to a significant event: the passing from
aquatic to terrestrial life.
By studying this, we can
understand certain aspects that are frequently present in the clinical
practice: Why do people with extreme respiraÂtory muscle weakness breathe as
frogs? (frog breathing); why do newborns with breathing diffiÂculties have
nasal flaring and expiratory grunting?; how is it possible that abdominal
muscles, which are typically expiratory, assist with inspiration in cases of
diaphragmatic paralysis?; why does the breathing pattern of respiratory failure
has less variability and becomes more rigid (apart from being fast and
superficial)? and, finally, is it possiÂble to imagine
a neutral pH that doesnât have the 7.0 value?; whatâs the use of this
knowledge, and how should gases in hypothermia be interpreted?
Water-to-land transition is one
of the most imÂportant and inspiring major transitions of verteÂbrate
evolution. The first fish appeared 438 million years ago, and the transition of
the tetrapod from water to land occurred around 375 million years ago; tetrapods were the main characters of this unique event:
they emerged from the water and breathed air. They were exothermic and
incapable of sustaining high levels of physical activity, and evolved into two
classes of vertebrates with high levels of maximal oxygen consumption: mammals
and birds.1 Terrestrial
ability appears to coincide with the origin of limbs; there was a coexistence
of aquatic features, such as the gills and tail fin with the limbs.2
CHANGES IN THE COMPOSITION OF GASES IN THE BIOSPHERE
Fluctuations in O2
and CO2 levels in the
biosphere have determined the way and means through which O2
was incorporated and CO2
was elimiÂnated. During the late Paleozoic era (around 300
million years), for a period of approximately 120 million years, the O2 level
increased to a maximum of 35% and then dropped abruptly to a minimum of 15% in
the Triassic. These changes doubled in the water and resulted in great events,
such as mass extinctions.
The highest CO2
levels occurred in the OrÂdovician and Silurian periods, whereas
in the Carboniferous, the CO2 level had
decreased to the current value (0.036%), although at the end of the Permian it
had increased by a factor of three. The structure and function of initial gas
exchangers were produced mostly by natural selection under environmental
conditions that were totally diffeÂrent from the current ones.3
THE AQUATIC AND TERRESTRIAL ENVIRONMENTS
The gasometric
composition of air is well-known by us. Oxygen in seawater derives mostly from
the air, so it is composed of the same gases of the atmosphere. Since the
oxygen is more soluble in water than nitrogen, there is a higher proportion of
oxygen in water than in the air. But from the point of view of dissolved oxygen
(molecular), the air has 210 cmÂģ of O2/L, and seawater contains only 9 cmÂģ/L. So, in general terms, dissolved oxygen is much
less abundant in water than in air.
The presence of macro- and
microalgae contriÂbutes directly with lighting to the oxygenation of seawater.
Only 1% of the light that has an impact on the surface of the sea reaches 200 m
of depth (photic zone).4
Thus, O2 availability
decreases significantly as water depth increases.
GAS EXCHANGE
The most significant modification
in gas exchange was produced as a consequence of the change in the structure of
teguments. Gas exchange in waÂter was produced by two routes: teguments and
incipient respiratory structures. With the passing to terrestrial life and the
appearance of scales (repÂtiles), teguments would provide protection against
desiccation, but would become less permeable to gas exchange.
In birds and mammals, the
feathers and fur proÂhibited skin gas exchange for good. That function would
then be exclusively performed by the lung, with air inlet through the mouth.
The alveolar air started to form, an intermediate station between atmospheric
air and blood, with remarkably stable gasometric
composition, temperature and humiÂdity. So the lungs of mammals evolved in
order to face a unique set of challenges:
â Ensure the sufficient supply of
inspired oxygen to all the pulmonary units whose exchange surÂface would reach
around 70-150 m2 in humans,
inside a confined thoracic space,
â That large gas exchange surface
had to be assoÂciated with a minimum barrier thickness, and,
â A microvascular
network had to be generated to accommodate the cardiac output of the right
ventricle and resist cyclic mechanical tensions, which increase many times from
rest to exercise.
The organs of the respiratory
system of various aquatic animals, such as the fish, are the gills and the
operculum. The opening and closing dynamics of the gills is controlled by the
cranial nerves that derive from the gill arches (trigeminal, facial, and glossopharyngeal).
The innervation of the gill area in frogs is developed from the facial,
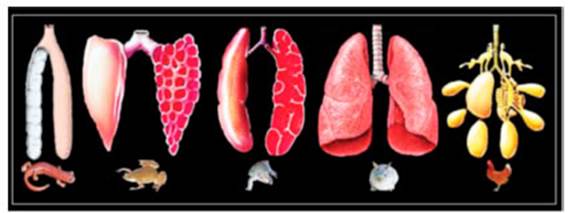
glossopharynÂgeal, and vagus cranial nerves.5 Figure 1
shows the morphological aspect of the lungs in vertebrates according to JN
Maina.3
Mammals and birds are the two
large classes of vertebrates that have high levels of maximum oxygen
consumption.1 A significant feature of these two groups is that even
though the physiology of the cardiovascular, renal, gastrointestinal, endocriÂne
and nervous systems shows many similarities, the lungs are radically different.6
Our perspective of self-aware
mammals could make us think that we have been more successful than birds.
Taking into account other aspects, it seems to be quite the opposite. West and
Watson proposed that the lung of birds is superior to that of mammals, and that
evolution in mammals has gone the wrong way:1
âĒ An important difference is the
fact that the ventilation of the gas exchange area (West resÂpiratory zone) has
a continuous flow pattern in birds, but shifts in mammals.
âĒ Birds move the gases through
convection, whereas mammals also need diffusion in the terminal airways.7
âĒ Birds have a more uniform
parenchyma with small terminal spaces that are largely intertwiÂned with the
capillaries, minimum membrane thickness and ultimately, more efficient gas
exchange.
âĒ Birds have separated the ventilatory and gas exchange functions, they seem to be
less vulÂnerable to bronchoaspiration and their
oxygen consumption in relation to their body weight is higher than that of
mammals.
For all those reasons, from a
structure-function standpoint, the bird lung is superior.1
Humans werenât the evolution goal (evolution doesnât have a
goal), still less the mammal lung. Evolution occurs gradually, not necessarily
towards more complex structures.8
In view of the great development
of his brain, the man is an acutely self-aware creature, more immensely capable
than any other animal of taking advantage of the individual and social
experience. While a climber struggles to get to the top of the Everest, the
geese are waiting for him, flying over his head.
THE RESPIRATORY PUMP
Evolution to terrestrial life
limited the gas exÂchange to the lungs, which evolved into a large exÂchange
surface exposed to a very much controlled alveolar air that had to be moved (ventilated)
in order to take air from the atmosphere. The respiÂratory pump, with its
different versions, was in charge of this.9
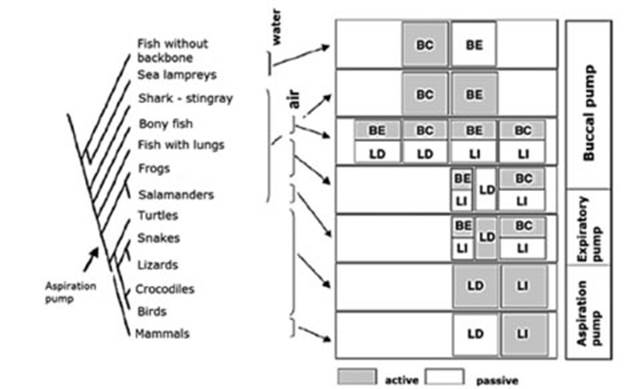
Figure 2 shows a graphic
representation (denÂdrogram) of several groups of
vertebrates in relaÂtion to the strategy used regarding the respiratory pump.9 We can
observe the change from a buccal pump driven
by branchiomeric (of the pharynÂgeal tract) and hypobranchial muscles (larynx, tongue, jaw) innervated by
cranial nerves to a thoracic-abdominal aspiration pump driven by axial
muscles innervated by spinal nerves with premotor neurons situated in the
ventral respiÂratory column.
The first steps in the evolution
of air-breathing were a modification of the behavior at the surface and changes
in the valves of the mouth/blowhoÂle/nostrils, the operculum and the glottis
(or their equivalent), that is to say, changes in the activation of the
muscles that expand or contract several openings. This allowed both aquatic and
air-breathing. Changes in the muscles of the resÂpiratory pump evolved later.10 So, the
evolution of respiratory mechanisms in vertebrates occurred from aquatic
ventilation promoted mainly by a buccal strength pump
to air ventilation driven mostly by a suction or aspiration pump. Only
mammals have a muscle diaphragm, of axial oriÂgin, innervated by spinal motor
neurons (phrenic nerve) and not by cranial nerves.
Between both ends of the buccal and aspiraÂtion pumps, there was the active
expiration (Figure 2, expiratory pump).9
This intermediate mechanism between the buccal pump of fish and amphibians and the aspiration pump
of reptiles, birds and mammals was barely known. It has been proven that many
amphibians use the axial muscles for active expiration along with the buccal pump for active inspiration. This suggests that
aspiration breathing evolved in two steps:
âĒ from buccal pumping alone to buccal
pumping for inspiration and axial muscles for expiration, and then
âĒ aspiration
breathing alone using axial muscles for both expiration and inspiration.
In mammals we can see a change in
the relaÂtive contributions of the chest wall distensibility
and resistance of the airflow to the respiratory effort (the first predominates
in birds and repÂtiles, the latter acquires greater
importance in mammals). We can also observe evolution into a muscle
diaphragm and a decreased need for active lung deflation as the system
returns to the state of equilibrium after inhalation (elastic recoil). Thus,
the functional residual capacity (FRC) is formed, that is the volume remaining
in the lungs at the end of passive expiration that represents the balance
between the forces expanding the lung and the ones that tend to collapse it
(respiratory rest).
In fish, amphibians and most
reptiles, there is certain division between the thoracic and abdomiÂnal
cavities. This partition is incomplete and not very efficient as a respiratory
pump. In developed reptiles and all mammals, there is a complete seÂparation of
the thoracic and abdominal cavities; this separation has a muscular layer in
the case of mammals. So, there is the diaphragm as a muscle and also aspiration
breathing (negative pressure).10
EVOLUTION OF ACID-BASE REGULATION
In humans, the PaCO2 is strictly controlled.
Throughout the day and night and even with the participation of other
non-respiratory functions, PaCO2 varies only
in a few mmHG. Furthermore, unlike the PaO2 that
decreases with age, the PaCO2 remains
constant for life. So any sustained deviaÂtion from the PaCO2
shall be seen as a significant alteration in homeostasis.11
What is the importance of such a
strict control of the PaCO2?
And, how is it achieved? The study of the
evolution of vertebrates from aquatic to terrestrial life and the capacity to
regulate body temperature allows us to understand why the PaCO2
has to be kept within such narrow limits.
In aquatic life under a
poikilothermic dynaÂmic, body temperature isnât constant, it varies according
to room temperature. The PCO2
suffers a great deal of variations and, although it is easily
removed (teguments permeable to CO2),
the main problem is oxygenation (the PO2
of water is lower than the atmospheric one), and due to various
reÂasons we will see later on, it is impossible to keep a constant pH value.
In terrestrial life, on
the other hand, the peripheral chemoreceptors (PQRs) stop working due to high
room PO2 (they
are sensitive to PaO2 below 60
mmHg), body temperature can be kept constant (homeothermia),
but now the sole CO2 elimination
route is expired air (due to the deveÂlopment of teguments that prevent
desiccation).
With a well-developed
thermoregulation caÂpacity and the precise regulation of PaCO2, the resulting, remarkably stable pH
allows mammals to maintain the ionization of the enzymes and the products of
internal metabolism, so that they are kept inside the
cell (the enzymes would escape from the cell if they lost ionization). This
strategy is called pH-stat: it is extremely important for homeotherms
to keep a constant pH (very narrow limits). Metabolic intermediates and enzymes
are completely ionized in the region that is close to neutral pH (neutral pH
from 7.0 to 25 °C) and have a low tendency to escape from the cell by going
through the membranes.
In other words, if the room pH
gets far off the ionization window of metabolic intermediates, these would lose
their charge and escape the cell. Hence the importance of
keeping the pH constant (pH-stat). This was elegantly expressed
as âthe importance of being ionizedâ.12
In order to understand why this
strategy isnât effective in poikilotherms, it is
important to menÂtion a fact that isnât usually taken into account: a body
temperature of 37 °C doesnât suggest a relaÂtionship between temperature and pH
(except in certain cases such as accidental or therapeutic hyÂpothermia): Temperature
changes the pH neutraliÂty value due to changes in the equilibrium constant of
water or kW (ion product of water). Thus, pure water has a neutral pH (value of
7.0) only at 25 °C, whereas at 10 °C and 35 °C, the neutral pH value is 7.27
and 6.98, respectively*.
In water, at low temperatures,
the internal pH of poikilotherms (fish, amphibians,
reptiles) tends to increase and consequently becomes far off the ionization
window of proteins and enzymes which as buffers can lose ionization but,
fortunately hisÂtidine with its α-imidazole group keeps a constant ionization; so, that
ionization keeps enzymes inÂside the cell and active regardless of temperature
variations. This is the so-called α-stat strategy.12-15
Histidine is a particular amino acid. It has three groups that are capable of
being charged: amine (pK 9.17), carboxyl (pK 1.82) and imidazole (pK 6.0);
and its net charge (or degree of dissociation) remains constant throughout the
whole tempeÂrature range and is the basis of Reevesâ α-stat theory.15
In fact, aquatic poikilotherms appeared long before terrestrial homeotherms and we had to find a homeostatic strategy when
teguments lost permeaÂbility to CO2.
The PCO2 constancy is a
success in terrestrial life and maybe it wouldnât have occuÂrred without the
dramatic complexity developed by the controlling structures of breathing.
But pH constancy in humans is
also the result of the interaction between multiple buffer systems in which
protein systems are found and the exact regulation of the bicarbonate/carbonic
acid system through ventilatory and renal control. It
is evident that all of this has been possible thanks to the evolution of the respiratory
centers.
EVOLUTION OF VENTILATION CONTROL
In the aquatic environment, the
PQRs of poikiloÂtherms are in charge of regulating
ventilation and the level of immersion, minute after minute. Their respiratory
centers consist of groups of relatively simple cells capable of generating a very
simple breathing pattern; for example, amphibians use only two groups of motoneurons that mediate ventilation. Far from acquiring
the phrenic nerve, the first nerves involved in the act of breathing were the
facial and glossopharyngeal.
With the passing to terrestrial
life, the strucÂtures that generate the respiratory rate were now oscillatory
neural networks of six groups of interconnected motoneurons,
and chemoreceptors sensitive to CO2 were
developed. The new neural circuits were stable but responsive to changes in the
levels of O2,
CO2,
pH, exercise, sleep, etc. Also, there needed to be coordination with phonation,
swallowing, airway reflexes, coughing, sniffing and locomotion. Thereâs also
long-term adaptation due to alterations in the thoracic cavity, the lung, and
the respiratory muscles caused by aging, weight gain or loss, pregnancy and
diseases. Finally, the new suprapontine structures
control the respiÂratory muscles voluntarily and in relation to a âevolutionary curiosityâ: emotions.
The model of respiratory centers
in mammals with the pneumotaxic and apneustic centers, the dorsal respiratory group and the
ventral respiraÂtory group, is already part of the history of meÂdicine. This
model arose from cross-section cuts of the trunk of sedated cats, decerebrated at the intercolicular
height, impaired, with or without vagus, and from the
observation of changes in breathing patterns and then from a total of six
transverse sections at different trunk levels.16, 17
At present, itâs impossible to
address the topic of the respiratory centers without speaking about the preBötzinger complex as a critical area for the
generation of the respiratory rate, and the retroÂtrapezoid
nucleus and parafacial respiratory group for the
generation of active expiration and the relationship with non-respiratory
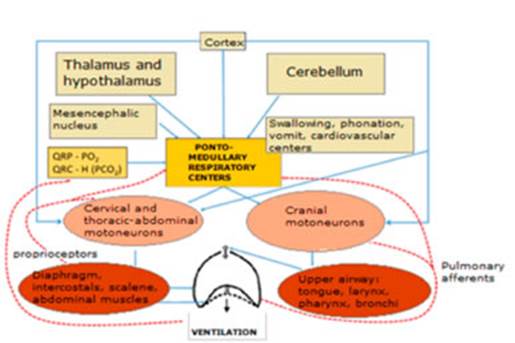
functions.17-20 Figure 3
shows the schematic organization of the respiratory system.
So, the evolution of respiratory control
from fish to mammals is characterized by higher complexity and is related to
the homeostasis of O2,
CO2,
pH and temperature.
â PQRs sensitive to hypoxia (PaO2 ≤ 60
mmHg and highly active under water) stopped functioning in terrestrial areas.
â When the constancy of the PaCO2 and the pH
were prioritized (pH-stat strategy), new areas were developed that were
very sensitive to CO2.
â The simple pacemaker cells gave
place to the preBötzinger complex, as well as
the paraÂfacial respiratory group and retrotrapezoid nucleus.23, 24
â Connections were developed with
non-respiraÂtory functions to coordinate respiration with phonation, swallowing
and vomiting.25
â We have a sort of homunculus
(primary motor area of the cortex, posterior frontal lobe, precenÂtral gyrus) that is
capable of voluntarily comÂmanding respiration over autonomic control.26
â Telencephalization
reached full development in humans. The cortex and subcortical/limbic system
structures (amygdala, cingulate gyrus, hippocampus,
etc.) mediate the emotions and influence respiration.
â The ponto-medullary
respiratory network, which produces the rhythmic central comÂmand for
respiration makes up stable, coordiÂnated and adaptable structures.
CONTROL OF VENTILATION AND ACID-BASE STATUS CLINICAL-EVOLUTIONARY
LESSONS
Only in developed reptiles and
all mammals, there is a complete separation of the thoracic and abdominal
cavities; this separation has a muscuÂlar layer in the case of mammals: the
diaphragm. Thus, aspiration breathing is developed in terrestrial and
aquatic mammals, but our gene pool seems to remember other evolutionary steps.
â Some people with neuromuscular
diseases and important respiratory muscle weakness use the frog breathing,
which allows the inlet of air at positive pressure. Frog breathing at
positive pressure (buccal pump), was
one of the first ventilatory modes of vertebrates.
â Nasal flaring and expiratory
grunting are signs of respiratory difficulties in newborns,27 babies and
toddlers.28 These
indicate an increase in breathing effort. This mechanism is unusual in adults.28 Various coordinated cranial nerves move those
structures and remember breathing with valves of the mouth/blowhole/nostrils
and the operculum and glottis (or their equivalent) like the first
vertebrates.
â In the case of bilateral
diaphragmatic paralysis, the abdominal muscles have an inspiraÂtory action.
Their action reduces the FRC, so in the next inspiration the air enters in a
passive way due to the state of equilibrium of the chest. The abdominal
muscles (expiratory pump) have an inspiratory function in certain
vertebrates.
â The variability of the
breathing pattern is lower in cases of acute respiratory failure. The use
of non-invasive ventilation reestablisÂhes variability and gets close to normal, with higher support levels.29
The rigid breathing pattern with poor variability reminds us
of poikilotherms. Evolution gained complexity and
variability but, with charge increase, the breathing pattern becomes more
rigid.
The use of general body
hypothermia for heart surgery has become a routine procedure. ConÂsequently,
the concept of neutral pH had to be reconsidered, and the experience of
millions of years of our ancestors, the poikilotherms,
had to be taken into account.
â Definition of neutrality (that
belongs to Arrhenius, 1889): it is not a âpH of 7.0â but the presence of
equal amounts of ions H+ and
OHâ. Since
temperature has the same effect on the concentration of each one of them,
neutrality is maintained regardless of temperature.30
â Regardless of the patientâs
temperature, arteÂrial gases are always analyzed at 37°C (it is the temperature
with which PO2,
PCO2 and pH
electrodes are measured). The gases of a hyÂpothermic patient are also analyzed
at 37°C, and if the PO2,
PCO2 and pH values
are within the normal range, the acid-base status of the patient will be
suitable for his/her temperature.31,
32
â The in vitro anaerobically
cooled blood of a mammal follows the acid-base status pattern of a poikilotherm. Physiologically speaking, to correct the
pH according to body temperature in hypothermia doesnât make any sense, because
the neutrality of pH also changes with temperature.
Given the amazing diversity of
living organisms, it is tempting to imagine an enormous amount of evolutionary adaptation
processes to solve the different challenges of living on earth faced by each
species. There are certain early development processes that share some crucial
factors, and some of the close and distant gene regulatory networks are
conserved.
In France, before the
Franco-Prussian War, there was a political satire about government changes that
said: âWe take the same and start againâ. Alphonse Karr (1808-1890)33 added his
famous phrase ÂŦplus ça change, plus câest la même choseÂŧ: the more things change, the more
they stay the same. According to the comparative biology, there seems to be
immutable principles even with evident superficial or morphological
differences. It is heartbreaking to think that we are the sole witnesses of
clinical findings that serve as testimony of the species that lived in remote
times and left us their evolutionary history, our evolutionary history.
Conflict of interest
The author has no conflict of interest to declare.
NOTES
* If the pH increases as
temperature falls, this does not mean that water becomes more alkaline at lower
temperatures. A solution is alkaline if there is an excess of hydroxyl ions
over hydrogen ions (that is to say, pOH > pH). As
long as the concentration of hydrogen ions and hydroxide ions stays the same,
water will still be neutral (pH = pOH), even if its
pH changes. The problem is that we are all familiar with 7.0 being the pH of
pure water (non-ionized), so anything else feels really strange. In order to
calculate the neutral value of pH it is necessary to know the Kw, which
increases with temperature, and if it changes, then the neutral value of pH
changes as well. At 25 °C the Kw (mol2
dmâ6) value is 1.00 x 10â14, the pH is 7.00 and the pOH
is 7.00. So, 7.00 + 7.00 = 14. At 10 °C we will have a
Kw value of 0.681 x 10â14,
pH of 7.08 and pOH of 7.08. So,
7.08 + 7.08 = 14.16. So, the pH of 7.00 and 7.08 at 25 °C and 10 °C,
respectively, is neutral because it has H+
= OHâ.34
REFERENCES
1. West JB, Watson RR, Fu Z. The human
lung: did evoluÂtion get it wrong? Eur Respir J 2007;29:11-17.
https://doi.org/10.1183/09031936.00133306
2. Dickson BV, Clack JA, Smithson
TR, et al. Functional adapÂtive landscapes predict terrestrial capacity at the
origin of limbs. Nature 2021;589:242-5. https://doi.org/10.1038/ s41586-020-2974-5.
3. Maina
JN. Comparative respiratory physiology: the funÂdamental mechanisms and the
functional designs of the gas exchangers. Open Access Animal Physiology 2014;53.
https://doi.org/10.2147/OAAP.S53213
4. Knoll AH, Bergmann KD, Strauss
JV. Life: the first two bilÂlion years. Philos Trans
R Soc Lond B Biol Sci 2016;5:371.
https://doi:10.1098/rstb.2015.0493.
5. Alonso DG. Desarrollo y diferenciación de las
branquias externas e internas en embriones y larvas de Bufo arenaÂrum:
análisis descriptivo y experimental. Tesis de Doctor. Facultad de
Ciencias Exactas y Naturales. Universidad de Buenos Aires. 2003.
http://digital.bl.fcen.uba.ar/Download/Tesis/Tesis_3581_Alonso.pdf
6. Hsia CCW, Hyde DM, Weibel ER. Lung Structure and the Intrinsic Challenges of Gas Exchange. Compr Physiol 2016;6:827-95. https://doi.org/10.1002/cphy.c150028.
7. West JB, Watson RR, Fu Z. The
human lung: did evoluÂtion get it wrong? Eur Respir J 2007;29:11-7.
https://doi.org/10.1183/09031936.00133306.
8. Smith HW. From
Fish to Philosopher. The American Biology Teacher 1962;24::445.
https://doi.org/10.2307/4440034
9. Milsom
WK. Evolutionary Trends in Respiratory MechaÂnisms. In: Poulin,
M.J., Wilson, R.J.A. (eds)
Integration in Respiratory Control. Advances in Experimental Medicine and
Biology 2008;605. Springer, New
York, NY. https://doi.org/10.1007/978-0-387-73693-8_51.
10. Fogarty MJ, Sieck GC. Evolution and Functional DifferentiÂation of the Diaphragm Muscle
of Mammals. Compr Physiol 2019;9:715-66.
https://doi.org/10.1002/cphy.c180012.
11. Arce SC, De Vito EL. More Breathing, Less Fitness: LesÂsons from Exercise Physiology in
Chronic Obstructive Pulmonary DiseaseâHeart Failure Overlap. Am J Respir Crit
Care Med 2017;196:1233-4. https://doi.org/10.1164/rccm.201707-1430ED
12. Rahn
H, Reeves RB, Howell BJ. Hydrogen ion regulation, temÂperature,
and evolution. Am Rev Respir Dis 1975;112:165-72.
13. Wilson RJA, Vasilakos K, Remmers JE.
Phylogeny of verteÂbrate respiratory rhythm generators: the Oscillator HomolÂogy
Hypothesis. Respir Physiol Neurobiol 2006;154:47-60.
https://doi.org/10.1016/j.resp.2006.04.007
14. Rahn
H. Body temperature and acid-base regulation. Pneumonologie
1974;151:87-94. https://doi.org/10.1007/BF02097155
15. Reeves RB. An imidazole alphastat hypothesis for vertebrate acid-base regulation:
Tissue carbon dioxide content and body temperature in bullfrogs. Respir Physiol 1972;14:219- 36. https://doi.org/10.1016/0034-5687(72)90030-8
16. Lumsden
T. Observations on the respiratory centres in the
cat. J Physiol 1923;57:153-60.
https://doi.org/10.1113/jphysiol.1923.sp002052
17. Stella G. On
the mechanism of production, and the physiÂological significance of âapneusisâ. J Physiol 1938;93:10-23. https://doi.org/10.1113/jphysiol.1938.sp003621
18. MacLarnon
AM, Hewitt GP. The evolution of huÂman speech: the role of enhanced breathing
control. Am J Phys Anthropol
1999;109:341-63. https://doi.org/10.1002/(SICI)1096-8644(199907)109:3<341::AID-AJPA5>3.0.CO;2-2
19. Sundin
L, Burleson ML, Sanchez AP, et al. Respiratory chemoreceptor function in
vertebrates comparative and evolutionary aspects. IntegrComp Biol
2007;47:592-600. https://doi.org/10.1093/icb/icm076
20. Feldman JL, Del Negro CA,
Gray PA. Understanding the Rhythm of Breathing: So Near, Yet So
Far. Annl Rev Physiol 2013;75:423-52.
https://doi.org/10.1146/annurev-physiol-040510-130049
21. Hilaire
G, Pásaro R. Genesis and control of the
respiratory rhythm in adult mammals. News Physiol Sci 2003;18:23-8. https://doi.org/10.1152/nips.01406.2002
22. Bianchi AL, Gestreau C. The brainstem respiratory netÂwork: an overview
of a half century of research. Respir Physiol Neurobiol 2009;168:4-12. https://doi.org/10.1016/j.resp.2009.04.019
23. Onimaru
H, Homma I. A novel functional neuron group for respiratory
rhythm generation in the ventral medulla. J Neurosci 2003;23:1478-86.
https://doi.org/10.1523/JNEUÂROSCI.23-04-01478.2003
24. Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on
respiratory rhythm. Nat Rev Neurosci 2006;7:232-41. 10.1038/nrn1871. https://doi.org/10.1038/ nrn1871
25. Evans KC, Shea SA, Saykin AJ. Functional MRI localization of central nervous
system regions associated with volitional inspiration in humans. J Physiol 1999; 520 Pt 2:383-92.
https://doi.org/10.1111/j.1469-7793.1999.00383.x
26. Nakayama T, Fujii Y, Suzuki K. et al. The primary moÂtor area for voluntary diaphragmatic motion identified by
high field fMRI. J Neurol 2004;251:730-5.
https://doi.org/10.1007/s00415-004-0413-4.
27. Silverman WA, Andersen DH. A controlled clinical trial of effects of water mist on obstructive
respiratory signs, death rate and necropsy findings among premature infants.
Pediatrics 1956;17:1-10.
28. Mas A, Zorrilla JG, García D, et al. Utilidad
de la detecÂción del aleteo nasal en la valoración de la gravedad
de la disnea. Med Intens
2010;34:182-7.
https://doi.org/10.1016/j.medin.2009.09.008
29. Giraldo
BF, Chaparro JA, Caminal P,
et al. CharacterizaÂtion of the respiratory pattern variability of patients
with different pressure support levels. Conf Proc IEEE Eng Med Biol Soc 2013;2013:3849-52.
https://doi.org/10.1109/EMBC.2013.6610384
30. Yartsev
A. Alpha-stat and pH-stat models of blood gas interpretation, In Deranged
Physiology. A free online resource for Intensive Care
Medicine. Last updated Tue, 02/08/2022.
https://derangedphysiology.com/main/cicm-primary-exam/required-reading/acid-base-physiology/Chapter%20115/alpha-stat-and-ph-stat-models-blood-gas-interpretation
(accessed 18 June 2022).
31. Williams JJ, Marshall BE. A Fresh Look at an Old Question. Anesthesiology 1982;56: 1-2.
https://doi.org/10.1097/00000542-198201000-00001
32. De Vito EL. Hipotermia [Hypothermia].
Medicina (B Aires). 1987;47:214-6. Spanish.
33. Karr A. Alphonse Karr: âPlus ça change, plus câest la même choseâ. LâHistoire
en citations,
https://www.histoire-en-citations.fr/citations/Karr-plus-ca-change-plus-c-est-la-meme-chose
2016 (accessed 18 June 2022).
34. Clark J. Temperature
Dependence of the pH of pure Water. Chemestry, Libre Texts. Last updated Aug
15, 2020. https://batch.libretexts.org/print/url=https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_TextÂbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Acids_and_Bases/Acids_and_Bases_in_AqueÂous_Solutions/The_pH_Scale/Temperature_Dependence_of_
the_pH_of_pure_Water.pdf (accessed 19 June 2022)