Autor Osejo-Betancourt, Miguel1, Moreno-RamĂrez, Carlos Ernesto2, Chaparro-Mutiz, Pedro3
1Specialist in Internal Medicine, Universidad Nacional Autónoma de Honduras, and in Pulmonology, Hospital Santa Clara, Bogotá, Colombia. 2Specialist in Epidemiology and Resident in Internal Medicine, Hospital Santa Clara, Bogotá, Colombia 3Specialist in Internal Medicine and Pulmonology, Hospital Santa Clara, Bogotá, Colombia.
https://doi.org./10.56538/ramr.FPKS7802
Correspondencia : Miguel Osejo Betancourt. E-mail: mosejob@unbosque.edu.co
ABSTRACT
Pulmonary alveolar proteinosis is
a clinical entity characterized by the accumulation of proteinaceous material,
rich in surfactant, mediated by reduced clearance by alveolar macrophages. In
adult patients, it is commonly associated with autoimmune phenomena resulting
in a deficiency of the granulocyte-macrophage colony stimulating factor, which
implies alterations in cell maturation and dysfunction, causing a decrease in
surfactant degradation and its accumulation in the alveolar space. Its
diagnosis poses a challenge to the clinician, based on the findings of
pulmonary function tests and the crazy paving pattern of the high-resolution
computed tomography of the chest, and is confirmed by obtaining the
proteinaceous material in the bronchoalveolar lavage. Given its rarity, the
ideal treatment remains to be elucidated, with whole lung lavage currently
being the corÂnerstone of treatment. We report an anecdotal case of a
41-year-old female patient sufÂfering from pulmonary alveolar proteinosis since
2011, who has required multiple whole lung lavages, with poor response to
these, with persistent dyspnea and supplemental oxygen requirement even though
she has performed the procedure, but with a progresÂsive tendency towards
improvement in the last 2 years. The lavage technique is not comÂpletely
standardized and its use in Latin America is still limited, which is why we
publish the protocol used in the Hospital Santa Clara of Bogotá,
Colombia.
Key words: Pulmonary alveolar proteinosis; Pulmonology; Protocol; Whole lung
lavage; Rare diseases
RESUMEN
La proteinosis alveolar
pulmonar es una entidad clínica caracterizada por la acumulaÂción
de material proteinaceo, con alta riqueza en
surfactante, mediado por una menor aclaración por parte de los
macrófagos alveolares. En pacientes adultos, comúnmente se asocia
a fenómenos autoinmunes que tienen como resultado una deficiencia del
factor estimulante de colonias de granulocitos y macrófagos, lo que
implica alteracioÂnes en la maduración y disfunción celular, lo
que provoca disminución de la degradaÂción del surfactante y su
acumulación en el espacio alveolar. Su diagnóstico corresÂponde a
un reto para el clínico, sobre la base de los hallazgos en pruebas de
función pulmonar, el patrón en “empedrado” (crazy
paving) en la tomografía computarizada de
tórax de alta resolución y que se confirma al obtener el material
proteínico en el lavado broncoalveolar. Dada
su rareza, el tratamiento ideal permanece por ser elucidado y en la actualidad
el pilar del tratamiento es el lavado pulmonar total. Reportamos un caso
anecdótico de una paciente de 41 años con proteinosis
alveolar pulmonar desde 2011, que ha requerido múltiples lavados
pulmonares totales, con pobre respuesta a estos, persistencia de disnea y
necesidad de oxígeno suplementario a pesar de realizar el procedimiento,
pero con tendencia progresiva a la mejoría en los últimos 2
años. La técnica del lavado no está completamente
estandarizada y su uso en América Latina es aún limitado, por lo
que publicamos el protocolo utilizado en el Hospital Santa Clara de Bogotá,
Colombia.
Palabras clave: Proteinosis alveolar pulmonar;
Neumología, Protocolo; Lavado pulmonar toÂtal; Enfermedades raras
Received: 12/20/2021
Accepted: 8/5/2022
INTRODUCTION
Pulmonary alveolar proteinosis
(PAP) is a pulÂmonary disease caused by the accumulation of surfactant in the
alveolar space mediated by a reduced clearance by alveolar macrophages. It was
first described in 1958 by Rosen et al.1, 2
The altered macrophage function
is the effect of the reduced availability of the granulocyte-macrophage colony stimulating
factor (GM-CSF) mediated by the production of autoantibodies against this
protein in up to 90% of adult patients. Other causes include mutations in the
GM-CSF receptors, hematologic disorders, infections, drugs and exposure factors
(silica, cellulose, heavy metals and some organic materials).1
PAP symptoms are non-specific,
with progresÂsive dyspnea as the main symptom. Lung function tests show reduced
diffusing capacity of the lungs for carbon monoxide (DLCO), and the spirometry
might show a restrictive pattern.3 The high-resoÂlution
chest tomography (HRCT) shows the crazy paving pattern, with ground glass
opacities superÂimposed on interlobular septal thickening that is
characteristic of this condition, though it may also be present in other diseases,
and in the cases of autoimmune etiology, the presence of antibodies against
GM-CSF confirms the diagnosis.4-6
The diagnosis must be confirmed
through bronchoalveolar lavage collecting whitish, milky proteinaceous material
with precipitating amorÂphous detritus; the microscopy showing oval,
acelÂlular bodies, basophilic in May-Grünwald-Giemsa Stain and positive
for PAS (Periodic acid Schiff) staining.1, 7
The treatment includes smoking
cessation and vaccination against influenza and pneumococcus for the prevention
of respiratory infections. The cornerstone of treatment for symptomatic
patients with reduced vital forced capacity (VFC), reduced DLCO or hypoxemia is
whole lung lavage. At presÂent there are other treatments such as inhaled or
subcutaneous GM-CSF, and for treatment-refractory patients, additional
interventions may be used, such as the use of rituximab, plasmapherÂesis or
lung transplantation, with studies of highly variable results.1, 8-10
Since it is a rare disease, there
aren’t any ranÂdomized clinical studies that standardize the whole lung lavage
technique; there are some descriptions in languages other than Spanish and
modifications in accordance with the Center’s experience, withÂout any
established protocols in Latin America. Taking that into consideration, the
objective of this review was to describe the protocol for the whole lung lavage
procedure that has been followed in the Hospital Santa Clara of Bogota, which
has been used for the treatment of several PAP patients in that institution;
and in comparison with the techniques described in the literature review, we
will briefly describe the experience of one case that was refractory to
treatment.
CASE REPORT
Female patient, 41
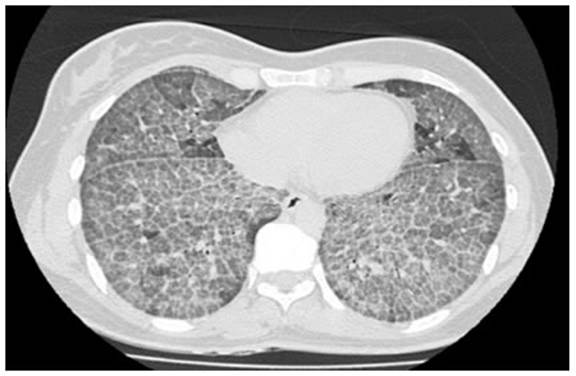
years old, who presented to the institution in 2011 with chronic dyspnea. Chest tomography showed crazy paving pattern (Figure 1). A bronchoscopy
was performed and proteinaceous material was collected. Gram, Ziehl Neelsen and
Grocott stains were performed, as well as cultures for bacteria, mycobacteria and
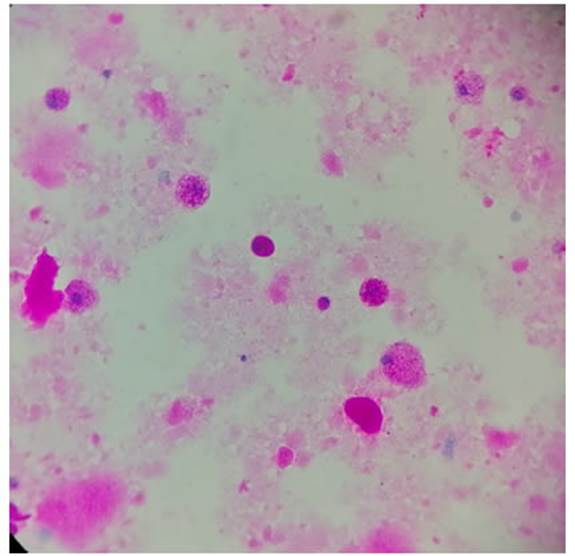
fungi, all with negative results but with PAS-positive stainÂing. PAP diagnosis
was confirmed through clinical symptoms, tomography, lavage findings, and
PAS-positive staining, without the need to perform a biopsy. The patient
proceeded with the whole lung lavage in the Pulmonology Department, with
unsatisfactory evolution, even though she had perÂformed multiple procedures.
The patient showed a partial response, and required lung lavages every 6 months
on average, 26 lung lavages in total (13 right, 13 left) since she was first
treated at the inÂstitution; and the protocol described in Table 1 was followed
at all times. No complications occurred during the procedure; during the
postoperative period, she only had some isolated fever spikes. The patient presented
pulmonary hypertension, with a 2018 echocardiogram showing calculated pulmonary
artery systolic pressure (PASP) of 70 mmHg. In September 2020, she went to the
emerÂgency department with exacerbation of respiratory symptoms, no fever,
oxygen saturation of 65% on admission, and the last lung lavage having been
done in August 2019. Once SARS-CoV-2 infection was discarded, a new lung lavage
was scheduled. During the lung lavage, a thick, yellowish proteinÂaceous
material was obtained (Figure 2), and then washed with 25 L. As there were no
complications, a new lavage was scheduled for the following week, and was also
performed without complicaÂtions. The patient was discharged with clinical
improvement, saturation 90% on supplemental oxygen. She reported significant
improvement with a few symptoms. New control echocardiogram in April 2021; PSAP
of 20 mmHg reported with normal systolic function. The dyspnea improved and as
regards the functional limitation, she no longer required oxygen for daily
activities (it was necessary only at night). She was admitÂted on November 2021
with dyspnea class 2 according to the mMRC (Modified Medical Research Council)
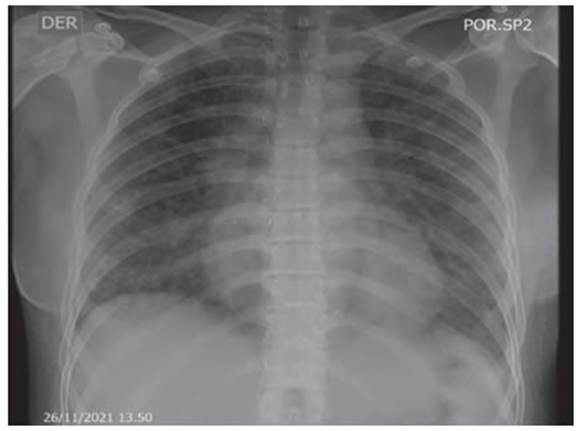
scale, saturation 88% on room air. Control chest X-ray (Figure 3) showÂing
bibasilar alveolar opacities with significant improvement compared to previous
tests. New whole lung lavage scheduled. Lavage performed in two sessions; the
second one, with 20 L. Fluid cleared completely (Figure 4) for the first time
in this patient. PAS staining requested (Figure 5) in the
last lavage. There weren’t any complications and the patient was
discharged without symptoms, with saturation 91% without supplemental oxygen.
She had a follow-up plan for external consultation, with tomographic control
and new echocardiogram to confirm previous findings.



METHODOLOGY
A narrative review was performed
of the bibliÂography using the PubMed, Google Scholar and ScienceDirect
databases between January 1st, 2014 and September 30th, 2021. Also, the search
of relevant bibliography was complemented with the review of the references of
articles selected by the authors. For the bibliography search we used the MeSH
Terms and selected the following as key words: «whole lung lavage» and
«pulmonary alveolar proteinosis» and their combinations, using AND and OR as
Boolean operators.
RESULTS
When searching and writing the
summary of the bibliography, articles were selected according to the
preferences of the topic to be discussed, includÂing review articles, case
reports, guidelines and protocols, observational studies and clinical trials.
DISCUSSION
In 1953, Dr. Benjamin Castle
described a patient with alveolar filling of proteinaceous material stained
with PAS staining, and in the following 5 years accumulated 27 patients with
the same findings, making the first report about this new disease which
subsequently received the name of pulmonary alveolar proteinosis. It is a
progressive, fatal disease with no suitable treatment, for which many therapies
have been tested with unfavorable results.11 In 1963, Dr. José Ramírez
Rivera used a transtracheal catheter placed in one lung at a time and instilled
100 ml saline solution aliquots at 50-60 drops per minute, four times a day, 2
to 3 times a week which, despite being a stressful and long process, improved
the opacities in the radiological images, the oxygenation and DLCO.11 In 1964, he
used a double lumen tube to instill 3 L of saline solution to an isolated lung
together with heparin or N-acetylcysteine and showed that the instillation of
large volumes is a safe procedure. During the following decades, the procedure
has been perfected and the whole lung lavage as we know it today was first
described in 1994.12
Indications and contraindications
The main indication is considered
to be dyspnea with functional limitation, worsening of radiologiÂcal images and
hypoxemia.9,
11 The
use of other clinÂical and paraclinical parameters to define whether or not a
lavage should be performed is variable according to the experience of each
center, and other causes of hypoxemia should be discarded, for example
pulmonary embolism or infection.12
The main contraindications to the
procedure include severe heart disease, findings of pulmoÂnary fibrosis or
sepsis.13 It is not
recommended for patients with untreated coagulopathy, especially in cases of
thrombocytopenia with platelet count below 50 000 /mmÂł or an INR (International
NorÂmalized Ratio) of more than 1.5; however, given the rarity of this
condition, there aren’t any ranÂdomized studies that guide the procedure, thus
explaining the high variability of the different institutions.11
In most centers, lung lavage is
preferably carÂried out in two sessions (one for each lung), with 1-3 weeks
in-between sessions; however, cases have been reported of bilateral lung
lavages performed in only one session.11
Most patients will require separate lung lavages, and even a
considerable number of patients will require only one proceÂdure to reach
spontaneous remission; that is why it is preferred as first-line treatment in
contrast to the administration of GM-CSF, which is more expensive and not
easily available in most Latin American countries.9, 12
The objective of lung lavage is
to remove as much proteinaceous material as possible, havÂing instilled the
least amount of solution, while reducing complications related to anesthesia
and post-procedure hospitalization.
Preparation
We suggest a suitable
preanesthetic assessment including pulmonary function tests and radioÂgraphic
control, and during the visit, advice should be given on the management of the
airway and of the double-lumen endotracheal tube.9, 12
For the induction and maintenance
of anesÂthesia use endovenous anesthetic agents to avoid leakage during the
lavage and contamination of the area, with fluid restriction to avoid fluid
overload, continuous hemodynamic monitoring, arterial gasometry and a patient
warming device so as to prevent hypothermia, especially in patients with
unstable cardiovascular disease.14,
15 In patients with significant polycythemia, a phlebotomy
could be considered before the procedure to reduce the risk of thromboembolic
complications.12
Protocol description
The procedure is carried out in
the operating room by trained staff including a pulmonologist, respiÂratory
therapy and an anaesthesiologist.11,
12 It is done with general anesthesia with neuromuscular
blockade, using a left double-lumen tube (because the placement of the right
tube is more complicated and might obstruct the bronchus of the upper lobe).
There must be an air column at both ends, and generally the anesthesiologist
does resistance tests to verify the position of the tube, which is then
confirmed with the bronchoscopy; this step is fundamental for the procedure.8, 11, 12, 14, 16
The procedure is carried out in
supine position because it is more comfortable and to prevent the double-lumen
tube from moving out of its place, a common complication of this procedure.
Some authors report cases of the procedure being performed with the patient
lying on a side, in the direction of the lung to be washed, to reduce the
probability of leakage towards the contralateral lung.11, 12
The lung to be washed is selected
with the supÂport of radiological images and confirmed during the procedure
with the evaluation of lung compliÂance: the most affected lung is the least
compliant. After the intubation, denitrogenation of both lungs is carried out
to avoid the formation of bubbles during the procedure through the
administration of a fraction of inspired oxygen (FiO2) of 100% for 5-15 min. During that time, the
saline solution is heated to 37 °C to reduce hypothermia, and approximately 20
L - 40 L are prepared for the procedure.8, 11, 12, 14, 16 A closed system is prepared with the Y-shaped
connector, with one end conÂnected to the saline solution bags and the other
one to the double-lumen tube towards the side of the lung to be washed, and the
other one, to the drainage system.14 When it is
ready, the instillaÂtion of the first aliquot begins slowly to avoid the
formation of bubbles and barotrauma, until the pressures are equal and no more
liquid enters the system.14 The inlet
valve is closed and the drain valve is opened (it may or may not be connected
to a suction system). The drain valve can be opened immediately after finishing
the instillation of the solution, because it seems to be as effective as
keeping it for a few minutes, and apparently it reduces absorption to the
systemic circulation and secondary hypervolemia.12
Once the drainage has begun,
chest percusÂsion is carried out to facilitate the mixture of the proteinaceous
material with the instilled solution. Percussion can be carried out with kinesiotherapy
or percussion equipment, like the one we use in our center, but it can also be
administered manuÂally. If done manually, it is exhausting for the staff and
the patient complains of more pain after the procedure.11, 12 The first drained material will be whitish and
milky, or from yellowish to reddish if there are microhemorrhages, and will
clear while aliquots are instilled.
The procedure is repeated with
aliquots of 1000 ml on average, the flow rate being deterÂmined by the infusion
system, and performing the percussion only during drainage. The amount of fluid
entering and leaving the system must be strictly calculated and monitored;
drips of more than 1000 ml might indicate system leakage toÂwards the
contralateral lung or the pleural space.8, 14 This could occur if the double-lumen tube
moves out of its place, and it could be necessary to replace it and confirm
with a new bronchoscopy; that is why it is important to pay attention to
bubbles going out from the contralateral lumen.8, 14
Cycles will be repeated until the
fluid drainage is as clear as possible. On average, most patients need 20 L per
procedure and may require up to 40 L.17
Physiological changes during whole lung lavage
During the filling phase, blood
is physiologically sent from the non-ventilated lung to the ventilated one due
to hypoxic pulmonary vasoconstriction and pressure changes induced by the
instillation of the solution. This change in the physiological shunt results in
higher oxygenation, since the heÂmoglobin saturation increases and, once
drainage is completed, oxygen saturation decreases again as a consequence of
airway pressure drop and pulmoÂnary perfusion towards the non-instilled lung.17 In addition,
fluid overload during the filling phase might increase pulmonary vascular
resistance, thus causing right ventricular overload, especially in patients
with pulmonary hypertension or left ventricular dysfunction.14
Post-procedure care
Once the procedure is completed,
the remaining liquid is absorbed from the lung. Then, ventilation and
recruitment of both lungs are carried out. If liquid is observed coming out
from the tube, it is absorbed. Subsequently, the lung that has been washed is
recruited and liquid is absorbed as needed.12, 14, 16
It is important to remember that
drained proteinaceous material is pulmonary surfactant lost during lavage, even
the amount necessary to maintain surface tension is lost, thus facilitating
alveolar collapse; that is why post-lavage recruitÂment is routinely done.11, 12
Taking into account the patient’s
conditions, extubation can be an option and transferring the patient to the
Intensive Care Unit to be surveilled during 12 to 24 hours. Patients with
severely comÂpromised oxygenation or hemodynamic instability are recommended to
replace the double-lumen tube with a conventional orotracheal tube after the
procedure and continue postoperative manageÂment in the Intensive Care Unit
with protective mechanical ventilation, with or without loop diÂuretics, using
the therapeutic strategies indicated for other causes of pulmonary edema.12
Technique variations
Some centers report the use of
the bilateral proceÂdure, which starts with the most affected lung and once the
liquid is clear, continues with the other. It is a much longer procedure, which
lasts up to 8 hours, then the orotracheal tube is replaced with a conventional
one and the patient is subsequently managed in the Intensive Care Unit.11 The adÂvantage of this method is that it reduces hospital
costs and the time until the patient is discharged, enhancing patient comfort
earlier. Silva et al pubÂlished in 2014 a series of 3 cases of bilateral lung
lavage, in accordance with their specific protocol.16
There are also some reports of
PAP patients with respiratory failure who didn’t tolerate single-lung
ventilation, so in those cases the procedure was performed with extracorporeal
membrane oxygenation.9,
11, 15, 16
Microbiological studies of
drained material can be conducted to study Nocardia, Actinomyces,
mycobacteria and fungi, such as Aspergillus and Cryptococcus,
because, given the dysfunction of alveolar macrophages, there is predisposition
towards these infections.17
The use of dynamic ultrasound
imaging has also been reported to guide the procedure and obÂserve the way in
which lung echogenicity changes, from being ventilated to achieving a
consolidation pattern after the solution has been instilled or evidencing
complete atelectasis after drainage. Echocardiographic guideline could reduce
pulÂmonary stress and help prevent volutrauma and barotrauma, and also check
for fluid leaks.18
Since 1988, Bingisser et al
described the use of manual ventilation during the procedure, and in 2012,
Bonella et al added intermittent percussion (both for instillation and
drainage) as a strategy to recruit a larger amount of proteinaceous mateÂrial
during the procedure with the least amount of solution.19
In 2021, Grutters et al modified the Bonella technique and
performed manual hyperinÂflation every 3 aliquots, with intermittent percusÂsion.
So, they found that the amount of solution necessary for performing the lavage
was reduced, with an average of 15 L with the largest amount of drained
material (91%) after 3 cycles with this maneuver.19
The work of Akasaka
et al in 2014 designed a mathematical model to predict the number of proÂteins
that were going to be removed during lung lavage, for the purpose of predicting
or modifying filling and drainage times. However, the calculaÂtion didn’t
modify the number of cycles or liquid retention time, compared to the standard
treatÂment, and the measurements of different proteins would increase procedure
costs.20
There is a report of a patient
with a bad reÂsponse to lavage, similar to our patient, that used treatment
combination, lung lavage with cycles of inhaled GM-CSF after the procedure for
up to 6 months, showing evident improvement; but this pharmacological
intervention isn’t easily available and its cost limits its administration
significantly, like in the case reported in this article.21
Segmental lavages are also
performed through flexible bronchoscopy in patients who don’t tolerÂate single
lung ventilation or pediatric patients, but with lower fluid volumes and
reduced effiÂcacy.13,
22
Follow-up and efficacy
Given the heterogeneous condition
of this disease, improvement of the various clinical parameters is different
and variable. In 2015, a retrospective study was carried out of 120 PAP adult
patients in China, 80 of which required lung lavage; they were followed-up for
8 years and after the procedure, the oxygen arterial pressure (PaO2), FVC, total
lung capacity, DLCO and distance travelled in the 6-Minute Walk Test (6MWT) all
improved: the most significant changes occurred in the DLCO, with an average
increase of 10 points, and in the 6MWT, with an average of 100 m, without any
dead patients in the follow-up period.23
In 2016, a meta-analysis was
published evaluatÂing the efficacy of the lavage; it included twelve studies
and reported significant improvement in the DLCO, FVC, PaO2 and forced expiratory volÂume on the first
second (FEV1), with no changes in the arterial pressure of the carbon dioxide
or the arterial oxygen saturation.24
Another 2020 report of 10
patients in India showed clear improvement in oxygenation, but only
stabilization or mild improvement in the other parameters of lung function.25 Another
report of fifty lung lavages conducted in a reference center in Thailand showed
improvement in oxygenation, DLCO, and radiographic findings, with 42% of
patients showing partial improvement and 47% complete improvement after the
procedure.26
It has also been found that
smoking alters the efficacy of the procedure, that is
why in smokers more lavages were necessary to achieve disease remission or
stabilization.17 Survival
after the procedure ranges from 63% to 94%, so it is part of the cornerstone of
treatment of PAP patients.17,
26
Complications
The most frequently reported
complications are: fever (18%), hypoxemia (14%), sibilance (6%), pneumonia
(5%), fluid leakage (4%), pleural effuÂsion (3.1%) and pneumothorax (0.8).9, 11, 13, 27 HyÂpoxemia is
particularly relevant, and is related to hospital readmission within 30 days
following the procedure in up to 5% of the cases, with patients reÂquiring
treatment with high FiO2 and
recruitment with positive end-expiratory pressure (PEEP), trying to avoid
barotrauma (pneumothorax).11,
28 Exacerbation
of the symptoms was also reported within the first 30 days following the
procedure, together with respiratory infections, though there was no
association with opportunistic infections.17, 28 Bad positioning of the double-lumen tube may
cause fluid leakage towards the other lung, but this rarely occurs with
highly-trained professionals and after confirmation with bronchoscopy. The
rapid instillation of large volumes might cause baroÂtrauma with
hydropneumothorax or significant pleural effusion, which could require
management with chest tube or thoracentesis.8, 11
Another important effect is
hypothermia. Body temperature should be monitored using physical media and the
aliquots should be heated before instillation, thus avoiding the appearance of
inÂtraoperative arrhythmia and other hypothermia-derived complications.8
It has been reported that up to
10% of patients resist whole lung lavage, with no significant imÂprovement, and
require more lavages plus other treatments.23
Our patient is included in that group, though she has shown
evident clinical improveÂment in the last 2 years of treatment.
CONCLUSIONS
Pulmonary alveolar proteinosis is
a rare disease, generally unknown to primary care physicians. This situation
could delay the diagnosis and, even though there are several treatment
alternatives, these are expensive and not easily available for most Latin
American countries. Cases have been reported of patients who respond to
treatment with stimulating factors, but whole lung lavage is still the
treatment standard and although the proÂcedure is expensive, it is
cost-effective, given the rapid improvement of the patient and long-term
maintenance. However, the procedure remains largely unknown, and many
physicians are afraid to use it due to the already mentioned implicaÂtions, and
the need for specific supplies, such as the double-lumen tube, and of staff
trained in the procedure and perioperative management; this indicates that it
is necessary to standardize some therapeutic strategies. For that reason, we
decided to write this protocol for the purpose of simplifyÂing the available
information, with an approach that can be easily replicated in most of the
Latin American territory.
Conflicts of interest
The authors have no conflict of
interest to declare in relaÂtion to writing or publishing this article.
REFERENCES
1. Kumar A, Abdelmalak
B, Inoue Y, Culver DA. Pulmonary alveolar proteinosis in adults:
pathophysiology and clinical approach. Lancet Respir Med
[Internet]. 2018;6:554–65.
https://doi.org/10.1016/S2213-2600(18)30043-2
2. Rosen SH, Castleman B, Liebow
AA, Enzinger FM, Hunt RTN. Pulmonary Alveolar Proteinosis.
N Engl J Med [Internet].
1958:258:1123–42. https://doi.org/10.1056/NEJM195806052582301
3. Inoue Y, Trapnell
BC, Tazawa R, et al. Characteristics of a Large Cohort of Patients with Autoimmune Pulmonary
Alveolar Proteinosis in Japan. Am J Respir Crit
Care Med [Internet]. 2008;177:752–62.
https://doi.org/10.1164/rccm.200708-1271OC
4. Ishii H, Trapnell
BC, Tazawa R, et al. Comparative Study of
High-Resolution CT Findings Between Autoimmune and
Secondary Pulmonary Alveolar Proteinosis. CHEST [Internet].
2009;136:1348–55.
https://doi.org/10.1378/chest.09-0097
5. Villar
A, Rojo R. Alveolar Proteinosis:
The Role of Anti- GM-CSF Antibodies. Arch Bronconeumol
[Internet]. 2018;54:601–2.
https://doi.org/10.1016/j.arbres.2018.03.017
6. Fisser
C, Hamer OW, Eiber R,
Pfeifer M, Lerzer C. Pflastersteine
in der Lunge [Crazy Paving Pattern of the Lung]. Pneumologie
[Internet]. 2019;73:49-53.
https://doi.org/10.1055/a-0767-7960
7. Burkhalter
A, Silverman JF, Hopkins III MB, Geisinger KR.
Bronchoalveolar Lavage Cytology in Pulmonary Alveolar Proteinosis. Am J Clin Pathol
[Internet]. 1996;106:504–10. https://doi.org/10.1093/ajcp/106.4.504
8. Misra
S, Das PK, Bal SK, et al. Therapeutic Whole Lung
Lavage for Alveolar Proteinosis. J Cardiothorac Vasc Anesth [Internet]. 2020;34:250-7. Available from:
https://doi.org/10.1053/j.jvca.2019.07.001
9. Iftikhar
H, Nair GB, Kumar A. Update on Diagnosis and Treatment of Adult Pulmonary
Alveolar Proteinosis. Ther Clin
Risk Manag [Internet]. 2021;17:701-10.
https://doi.org/10.2147/TCRM.S193884
10. Soyez
B, Borie R, Menard C, et al. Rituximab for
auto-immune alveolar proteinosis, a real life cohort study. Respir Res [Internet]. 2018;19:74. https://doi.org/10.1186/s12931-018-0780-5
11. Awab
A, Khan MS, Youness HA. Whole lung lavage-techniÂcal
details, challenges and management of complications. J Thorac
Dis [Internet]. 2017;9:1697–706.
https://jtd.amegroups.com/article/view/13803/11597
12. Abdelmalak BB, Khanna AK,
Culver DA, Popovich MJ. Therapeutic Whole-Lung Lavage for Pulmonary Alveolar
Proteinosis: A Procedural Update. J Bronchology
Interv Pulmonol [Internet].
2015;22. https://doi.org/10.1097/LBR.0000000000000180
13. Campo I, Luisetti M, Griese M, et al. Whole lung
lavage therapy for pulmonary alveolar proteinosis: a
global surÂvey of current practices and procedures. Orphanet
J Rare Dis [Internet]. 2016;11:115. Available
from: https://doi.org/10.1186/s13023-016-0497-9
14. Mata-Suarez SM, Castro-Lalín
A, Mc Loughlin S, de Domini
J, Bianco JC. Whole-Lung Lavage- a
Narrative Review of Anesthetic Management. J Cardiothorac Vasc
Anesth [Internet]. 2022;36:587–93.
https://doi.org/10.1053/j.jvca.2020.12.002
15. Tempe DK, Sharma A. Insights
into Anesthetic ChalÂlenges of Whole Lung Lavage. J Cardiothorac
Vasc Anesth [Internet].
2019;33:2462–4.
https://doi.org/10.1053/j.jvca.2019.04.033
16. Silva A, Moreto
A, Pinho C, Magalhães
A, Morais A, Fiuza C.
Bilateral whole lung lavage in pulmonary alveolar proteinoÂsis
– A retrospective study. Rev Port Pneumol [Internet].
2014;20:254–9.
https://doi.org/10.1016/j.rppneu.2014.04.004
17. Jouneau
S, Ménard C, Lederlin
M. Pulmonary alveolar proteinosis. Respirology [Internet]. 2020;25:816–26.
https://doi.org/10.1111/resp.13831
18. Sigakis MJG, de Cardenas JL. Lung Ultrasound Scans DurÂing Whole Lung Lavage. CHEST
[Internet]. 2021;159:e433– 6.
https://doi.org/10.1016/j.chest.2020.06.089
19. Grutters LA, Smith EC,
Casteleijn CW, et al. Increased Efficacy of Whole Lung Lavage Treatment in
Alveolar Proteinosis Using a New Modified Lavage Technique. J Bronchology Interv Pulmonol [Internet]. 2021;28(3). https://doi.org/10.1097/LBR.0000000000000741
20. Akasaka
K, Tanaka T, Maruyama T, et al. A mathematical model to
predict protein wash out kinetics during whole-lung lavage in autoimmune
pulmonary alveolar proteinosis. Am J Physiol - Lung Cell Mol Physiol [Internet]. 2014;308:L105– 17. https://doi.org/10.1152/ajplung.00239.2014
21. Yu HY, Sun XF, Wang YX, Xu ZJ, Huang H. Whole lung lavage combined with
Granulocyte-macrophage colony stimulating factor inhalation for an adult case
of refractory pulmonary alveolar proteinosis. BMC Pulm med [Internet]. 2014;14:87. https://doi.org/10.1186/1471-2466-14-87
22. Gay P, Wallaert
B, Nowak S, et al. Efficacy of Whole-Lung Lavage in Pulmonary Alveolar
Proteinosis: A Multicenter International Study of GELF. Respiration
[Internet]. 2017;93:198-206.
https://doi.org/10.1159/000455179
23. Zhao YY, Huang H, Liu YZ,
Song XY, Li S, Xu ZJ. Whole Lung Lavage Treatment of
Chinese Patients with AutoimÂmune Pulmonary Alveolar Proteinosis:
A Retrospective Long-term Follow-up Study. Chin Med J (Engl)
[Internet]. 2015;128:2714-9.
https://doi.org/10.4103/0366-6999.167295
24. Zhang HT, Wang C, Wang CY,
Fang SC, Xu B, Zhang YM. Efficacy
of Whole-Lung Lavage in Treatment of Pulmonary Alveolar Proteinosis.
Am J Ther [Internet]. 2016;23:e1671- 9. https://doi.org/10.1097/MJT.0000000000000239.
25. Marwah
V, Katoch CDS, Singh S, et al. Management of primary
pulmonary alveolar proteinosis: A multicentric experience. Lung
India [Internet]. 2020;37:304-9.
https://doi.org/10.4103/lungindia.lungindia_401_19.
26. Kaenmuang
P, Navasakulpong A. Efficacy of whole lung lavage in
pulmonary alveolar proteinosis: a 20-year exÂperience
at a reference center in Thailand. J Thorac Dis
[Internet]. 2021;13:3539-48.
https://doi.org/10.21037/jtd-20-3308
27. Hunter Guevara
LR, Gillespie SM, Klompas AM, Torres NE, Barbara DW. Whole-lung lavage in a patient with pulmonary
alveolar proteinosis. Ann Card Anaesth
[Internet]. 2018;21:215-7.
https://doi.org/10.4103/aca. ACA_184_17.
28. Smith BB, Torres NE, Hyder JA, et al. Whole-lung Lavage and Pulmonary Alveolar Proteinosis: Review of Clinical and Patient-centered
Outcomes. J Cardiothorac Vasc Anesth [Internet]. 2019;33:2453–61. https://doi.org/10.1053/j.jvca.2019.03.047