Autor : Pascansky, Daniel1-2, SĂvori, MartĂn1-2, Capelli, Luciano1-2
1Pneumophthisiology Unit, Hospital de Agudos Dr. J. M. Ramos MejĂa, Autonomous City of Buenos Aires. 2Centro Universitario NeumonologĂa Dr. J. M. Ramos MejĂa. Faculty of Medicine, University of Buenos Aires. Argentina.
https://doi.org./10.56538/ramr.LYBB1788
Correspondencia : Daniel Pascansky, PneumoÂphthisiology Unit, Hospital General de Agudos Dr. J. M. Ramos MejĂa, Urquiza 609, 1221 Buenos Aires, Argentina e-mail: vdpascan@gmail.com
ABSTRACT
There is not information about
the annual and structure of costs of a hospitalization of COPD exacerbation in
our country actually.
Objective: To determine the structure of direct costs in hospitalized patients due
to COPD exacerbations in a public hospital of Buenos Aires in 2018.
Methods: Patients hospitalized of COPD exacerbation (GOLD) in 2018 were analyzed
in our hospital. Direct costs were determined (financier perspective), due to
modulation of the Health Ministry of Buenos Aires City Government, stratified
by Intensive Care Unit hospitalization and in room at June 2021, in dollars
(dol.), parity at June 30th 2021 was 1 dollar = 101,17$ (price Banco
Nación).
Results: 26 patients were hospitalized: age 64 ± 9.56 years, male gender 73%, 61%
actual smokers and 39% ex-smokers (101.8 ± 47.1 pack-y, social health assurance
31% (n = 8); FEV1%
31 median (23-42) and FEV1/FVC
0,46 ± 0,12. Ward length of hospitalization (median) was 1 day (1-1,75), 9 days
in room (4-12), 13 days in UCI (11- 29,5) with mortality rate 23% (n = 6).
Final direct cost by patient was
1462,62 dol, median (IQR
25%-75%,763,85-2915,95),at 162,44 dol./day/patient. Total cost (n = 26)
was 117 480 dol. UCI cost was median 9898,28
dol./patient (IQR 25%-75%, 6700,94-35 780,25). Final UCI total cost (n = 3) 75 942,3 dol.
Conclusion: Patients with COPD exacerbation hospitalized were mainly males, sixty
years old, heavy smokers and severe airway obstruction. With financier
perspective, direct cost of hospitalization was 1462 dol./patient,
almost seven times higher in UCI. Disease management program must be
implemented to manage COPD, to identify patients at risk, to educate and to
assure access to drugs.
Key words: COPD, Exacerbations, Hospitalizations, Costs, Expense
RESUMEN
No existe información sobre la estructura y costos
anuales de una hospitalización por agudización de la EPOC en
nuestro país actualmente
Objetivos: Determinar la estructura de costos de los pacientes hospitalizados por EPOC
reagudizada en un hospital público de la Ciudad Autónoma de
Buenos Aires (CABA) en el año 2018.
Materiales y métodos: Se evaluaron pacientes con EPOC reagudizada (GOLD), inÂternados durante 2018
en nuestro hospital. Se determinaron costos directos (perspecÂtiva del
financiador), según costos de medicamentos y la modulación de
internación clínica y Unidad de Terapia Intensiva (UTI) del
Gobierno de CABA a junio de 2021, valor dólar Banco Nación al 30
de Junio 2021 de $101,17.
Resultados: Se internaron 26 pacientes, edad 64 ± 9,56 años, masculino 73%, 61%
tabaquistas actuales y 39% extabaquistas (101,8 ±
47,1 paq.-año), seguro social 31%, FEV1% 31 mediana
(23-42) y FEV1/FVC 0,46
± 0,12. La duración de internación fue: guardia 1 d (1-1,75);
piso, 9 d (4-12); y UTI, 13 d (11-29,5), con mortalidad 23% (n = 6).
El costo final fue 1462,62 dólares/paciente,
mediana (RIQ 25%-75%,763,85-2915,95), 162,44 dólares/d/paciente, y el
costo total (n = 26) fue USD 117 480. El costo de UTI fue 9898,28
dólares/paciente, mediana (RIQ 25%-75%, 6700,94-35 780,25). El costo
total (n = 3) fue USD 75 064,11.
Conclusión: Los pacientes con EPOC reagudizada que se hospitalizan son en su
mayoría hombres, más de 60 años, alta carga
tabáquica y obstrucción grave. El costo directo desde la
perspectiva del financiador fue de USD 1462 por paciente; el costo del paciente
que se hospitaliza en UTI fue casi siete veces superior. Se deben instruÂmentar
programas sistematizados de manejo de la EPOC para identificar pacientes con
factores de riesgo, educar y permitir acceso a la medicación.
Palabras claves: EPOC, Exacerbaciones, Hospitalizaciones, Costo directo, Gastos
Received: 5/2/2022
Aceptado: 11/3/2022
Chronic obstructive pulmonary
disease (COPD) is an important public health problem because there is growing
evidence of the increase in diffeÂrent epidemiological parameters of
considerable concern.1 Smoking is
the main cause of COPD.2,
3 The
smoking prevalence has been decreasing from rates of around 40%, at the
beginning of this century, up to near 22% of the general population, according
to the national Health Survey of our couÂntry.4
The age of smoking initiation in our country is 14 years; the age
of regular consumption is 18, and a tendency to increase consumption is obserÂved,
particularly in the areas of young people of low income.2, 4 Clearly, smoking cessation is still the main
therapeutic intervention that improves morÂbidity and mortality.2, 3 It is
important to highlight the underdiagnosis reflected in several European
studies, of around 75% of the total number of patients with COPD.5-7 In the
PLATINO study of five cities of Latin America, 82% of COPD patients were
unaware that they had this disease, with 77.4% in the Argentinian prevalence
study, EPOC.AR.8,
9 COPD
prevalence in the urban general poÂpulation of Argentina is 14.3%, but in a
sample of a population of more than 40 years with exposure to tobacco (PUMA
study) it is higher (29.6%), thus estimating between 2.5 and 3 million patients
with COPD.10 With
regard to mortality, according to the WHO, it is still the third cause of
death, and 80% occurs in countries of low- or middle- income.11
Preliminary studies about COPD mortality in our country show 112%
growth compared to 1980, and reaching almost 27 deaths per 100,000 inhabitants
in 1998, approximately 5000 deceased, especiaÂlly women.12
Recently, the National Institute of Respiratory Diseases “Dr.
Emilio Coni” reported approximately 30,000 annual hospitalizations in part of
the public sector in 2015.13
With respect to the morbidity,
COPD is the fifth cause of hospitalization in Argentina in individuals older
than 60 years.3 In the United
States of North America, hospitalizations have increased between 1978 and 1994
from 259,000 to 500,000 per year, especially in individuals older than 65
years.15 As a
disabling disease, COPD will go up from the current 12th place to the 5th place
throughout the world.16 The
increasing cost of chronic diseases has required the analysis of the impact of
the diseases on the health system, the cost structure, and the approaches that
tend to optimize the system, since the demand is always growing and not satisfied,
and health-related costs increase year after year. The progressive increase in
life expectancy, the use of diagnostic techniques that are becoming more and
more costly, and more expensive treatments had to be weighted in relation to
the savings generated by the reduction in the use of health resources, the
increase in survival and the improvement in different health markers, such as
quality of life.16-22 The National
Heart, Lung, and Blood Institute (NHLBI) of the United States acknowledged USD
23.9 billion in COPD expenses, 61.5% for direct costs. The cost per person was
USD 1,522 per person, per year, three times higher than the cost of asthma and
2.5 times higher than not-COPD.15,
21 The
highest costs were the direct costs for hospitalization and visits to the
emergency department (72.8%).22
In a study on emphysema costs in Great Britain, from the National
Institute for Health of the United Kingdom, Guest et al determined an
expenditure of ÂŁ19 million (approximately USD 34 million) to treat 134,000 emphysema
exacerbations (direct costs): 50% for hospitalization costs to treat 3% of
total exacerbations.23 The average
hospitalization cost was USD 3,600 versus USD 128 for exacerÂbations treated on
an outpatient basis. For that reason, strategies have been created to reduce
the number of hospitalizations and their length.23, 24 The average hospital
stay was 9.9 d. The mean estimation per hospitalization was ÂŁ 3,000 (USD 5,700)
as opposed to ÂŁ 100 (USD 190) for outpatient treatment.24
There are other multiple U.S and European publications about the
cost structure of COPD, all of them with the common denominator of the high
percentage of hospitalization due to exacerbation and oxygen therapy, which
severely impacts on the total cost of this disease.25-44
In Argentina, there is only one
publication from twenty years ago about the impact on directs costs of
hospitalizations for exacerbated COPD: 33 patients in 1999, in a public
hospital of the GoverÂnment of the City of Buenos Aires.36
The cost per discharge was USD 2,451, with an average stay of 15
d, at USD 163 per hospitalization day.36
The purpose of this study was to
describe the direct cost per each hospitalization for COPD exaÂcerbation and to
determine the cost structure in a public hospital of the city of Buenos Aires
in 2018.
MATERIALS AND METHODS
We reviewed the medical records
of patients hospiÂtalized for exacerbated COPD in every area of the Hospital
General de Agudos Dr. J. M. Ramos Mejía of the Autonomous City of Buenos
Aires (CABA) from January 1st, 2018 until December 31sr, 2018. Adults older
than 18 years were included. Patients with COPD (GOLD definition: FEV1/FVC
ratio < 0.70 and postbronchodilator predicted FEV1 < 80%2), older than 40 years, with smoking history
(more than 20 packs of cigarettes per year). PaÂtients with history of other
respiratory diseases were excluded.
Direct costs were determined from
the funder’s perspective, taking into account the cost of mediÂcations and the
modulation of the Government of the City of Buenos Aires (GCBA, for its acronym
in Spanish) for hospitalization in the intensive care unit (ICU) and regular
ward in June 2021. The values of the government’s modulation for public
hospitals by June 2021 were: ARS 14,143 (USD 139) for hospitalization in the
ward, per patient, per day; non-critical emergency room, ARS 2,957 (USD 30);
emergency room including tests, ARS 5,231 (USD 51.5); critical emergency room,
ARS 33,070 (USD 325); at the ICU, without mechanical respiratory assistance
(MRA), ARS 29,527 (USD 290); and with MRA, ARS 33,070 (USD 325) per patient,
per day.45 Each
module had certain pre-established number and type of service (biochemistry,
imaging, electrocardiogram, spiroÂmetry, MRA, oxygen, disposable material,
drugs, etc., apart from the rate that depends on salaries, taxes and charges,
administrative fees, equipment amortization, food and laundry costs, etc.). WheÂnever
a patient had any additional consultation, diagnostic practice or treatment
(for example, drugs) outside modulated fees, the cost was calÂculated from the
funder’s perspective according to the KAIROS pharmaceutical vademecum and the
list of services of the GCBA nomenclature.46 Given the varying peso/dollar parity,
results shall be reported in dollars. The exchange rate used to calculate the
cost in dollars was based on the quote of the Banco Nación on June 30th,
2021 ($ 101.17 = USD 1).
The eosinophilia value was
obtained before the administration of systemic corticosteroids at the
laboratory of the emergency department.
The spirometry was performed the
last hospitaÂlization day, before discharging the patient.
Descriptive statistics were used.
For quantiÂtative variables with non-Gaussian distribution, we used the median
as central measure and the interquartile range 25%-75% (IQR 25%-75%) as
dispersion measure. In order to have a gaussian distribution, we used the mean
as central measure and standard deviation as dispersion measure, and for
qualitative variables, we used percentages.
RESULTS
During 2018, 26 patients were
hospitalized: 23 in the regular ward and 3 in the ICU. Table 1 shows the
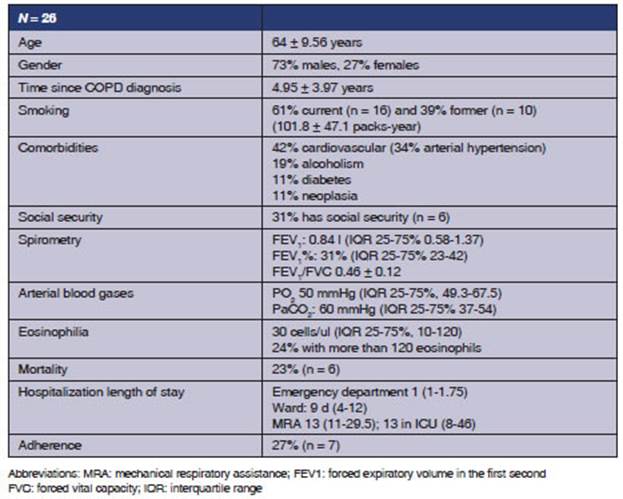
demographic characteristics.
Most patients (n = 18 patients
[70%]) belonged to the programmatic area of the hospital. From all the
patients, only 38.6% (n = 10) had been treated in our hospital before
their consultation. Adherence to treatment before hospitalization was poor
(27%).
Upon admission, 50% (n =
13) of the patients were receiving treatment with short-action beta- 2
adrenergic bronchodilators; 23% (n = 6), with combination of
short-action beta-2 adrenergic and anticholinergic bronchodilators; 15% (n =
4), with a combination of long-action beta-2 adrenergic bronÂchodilators and
inhaled corticosteroids; and 11% (n = 3), with long-action
anticholinergic bronchodilators. None of the patients were treated with triple
therapy.
Three of the 26 patients (11.5%)
were hospitaÂlized twice.
Direct cost analysis
The final direct cost per
hospitalized patient in the regular ward was USD 1,462.62 (IQR 25%- 75%,
763.85-2,915.95), which considering the 26 hospitalized patients, gives a total
direct cost of USD 117,480, that is, USD 162.44 per patient, per day.
The final direct cost per
hospitalized patient at the ICU was USD 9,898.28 (IQR 25%-75%,
6,700.94-35,780.25). Taking into account the fact that only three patients were
hospitalized at the ICU, there was an important dispersion of the total direct
cost per patient. The total amount spent for three patients was USD 75,064.11.
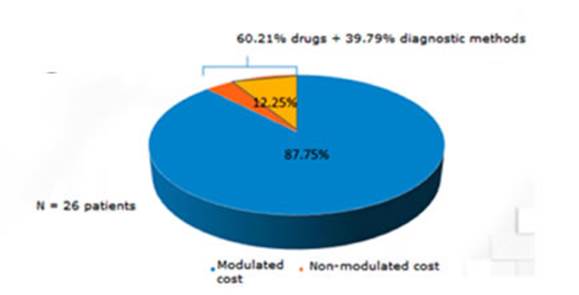
With regard to the direct cost
structure, 87.75% of it had been considered inside the clinical module of the
GCBA. However, 60.21% of the rest (12.25%) was related to drugs not included in
the module, and 39.8% to diagnostic practices not included in the module
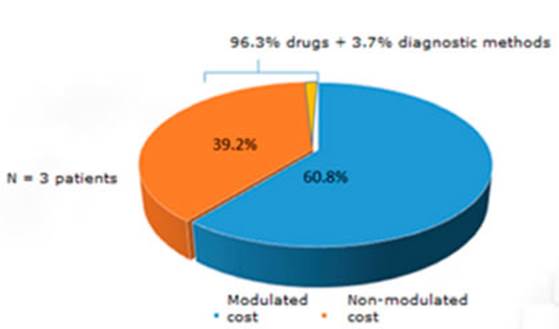
(Figure 1). But, if we consider ICU hospitalization, 39.2% of the direct cost
wasn’t modulated (three times higher than patients hosÂpitalized
in the regular ward). The cost of drugs (specially
antibiotics) was the main cause of non-modulated direct cost (96.3%) (Figure 2).
DISCUSSION
The direct cost of exacerbated
COPD hospitalizaÂtion in a public hospital in the Autonomous City of Buenos
Aires has been established. The sample consisted of 26 patients, mostly males
aged in their 70s, with recently diagnosed disease, high smoking load and
severe obstruction of the airflow. The cost in the regular ward was USD 1,462
per patient, and almost seven times higher in the ICU. Patients hospitalized in
the regular ward were included in the expected cost module, whereas those from
the ICU had a higher percentage outside the module, due to the use of high-cost
drugs. The pharmaÂcological treatment profile of most patients in public
hospitals in CABA is not included in the recommendations of current guidelines,
since it is mainly based on the use short-acting bronchoÂdilators, surely due
to the difficulty with access to medication and poor adherence to follow-up.
For the European
Union, total annual direct costs of respiratory diseases account for 6% of the
total healthcare budget; COPD represents 56% (38.6 billion Euros).2, 47
In United States, total annual direct costs for COPD are calculated in USD 29.5
billion, and indirect costs, USD 20.4 billion.2, 48 The highest proportion is
related to COPD exacerbation care, which determines a diÂrect relationship with
the severity of the disease. Probably in underdeveloped countries the indirect
cost is higher than the negative impact on the direct cost.2, 36-43
Given the fact that
mortality only offers a limited perspective of the impact of a disease on the
human being, Murray et al designed a DALY indicator (Disability-Adjusted Life
Year) that was analyzed in the Global Burden of Disease Study; it is the
sum of years of life lost due to premature death adjusted by the severity of
the disease and concomitant impairment of quality of life.49 So, in
2005 COPD was the eighth cause of DALY lost in the world, and it was estimated
that in 2013 it was the fifth cause worldwide, though second in the United
States, after coronary disease.49
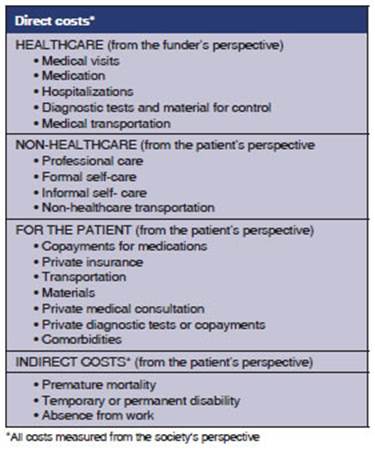
The Asthma Management
Spanish Guidelines (GEMA, for its acronym in Spanish) have deterÂmined the
different elements of the direct and indirect cost of asthma, which can be
extrapolated to another chronic obstructive disease, such as COPD. The
Guidelines recommend forty seven features to be used in a cost study (Table 2).50
Our study implements those recommendations. A mixed
methodology has been used to determiÂne direct costs: modulation of costs
provided by the GCBA (top-down method) and, apart from reviewing all medical
records, covering patient’s consumption outside the modulation (bottom-up
method). In our study, direct primary data have been collected from the medical
records, and that is very valuable information.50 As
we have already mentioned, we conducted the cost study from the funder’s
perspective (GCBA) in the environment of a public general acute care hospital,
thus, the conclusion will only be extrapolated to that health system. Cost
comparison between countries or diÂrect extrapolation are not recommended,
because cost structure and health systems vary from one country to another,
although it may help by giving us an idea of the magnitude of the problem and
the qualitative strength of each variable.50
Bilde et al published
a study on Medicare spenÂding per person, comparing COPD patients with non-COPD
patients. They determined that the cost was USD 8,482 versus USD 3,511 (2.5
times higher). 50% of the cost of COPD is consumed in 10% of the patients.51
Other authors have reported the economic impact of
COPD on the North AmeriÂcan Health System. The average days/bed was 7.75 d, but
with higher rates of rehospitalization and higher expenses. The average cost
was USD 6,469 per patient (direct costs), 68% of which belonged to
hospitalizations.48, 52 It has already been said that the National
Heart, Lung, and Blood InstiÂtute (NHLBI) of the United States acknowledged a
COPD cost of USD 23.9 billion: 14.7 billion were direct costs (61.5%) and 9.2
were indirect costs (38.5%). The cost per person was USD 1,522 per year, three
times higher than the cost of asthma and 2.5 higher than non-COPD patients.15,
21 The hospitalization index was 21.2 patients
for every 1000 persons. The highest direct costs were from hospitalization and
visits to the emergency department (72.8%). The rest was divided: 15% for
outpatient visits and 12.2% for cost of drugs. But the distribution of COPD
expenses was very disproportionate: 10% of patients used 73% of the total
expenses.15, 21 The increase in the number
of hospitalizations was observed in patients older than 45 years. The NHLBI
report includes a comÂparison within respiratory diseases, between the
distribution of direct and indirect costs of COPD with asthma, influenza, pneumonia,
tuberculosis and lung cancer.15 COPD uses twice the amount of
asthma, and compared to influenza, lung cancer or pneumonia, it shows a higher
proportion of direct costs.15 Guest et al, in the first study
on emphyÂsema costs in Great Britain, from the National Institute of Health of
the United Kingdom, deterÂmined a cost of ÂŁ 19 million (approximately USD 34
million) to treat 134,000 exacerbations due to emphysema (direct costs): 50%
for hospital costs to treat 3% of total exacerbations.23 The average cost per
hospitalization was USD 3,600 versus USD 128 in exacerbations treated on an
outpatient basis. This allowed the creation of strategies to reduce the number
of hospitalizations and their length.17 Again, Guest et al conducted
another study, similar to the previous one, but taking into account the general
COPD costs in the National System of Health of the United Kingdom. An amount of
ÂŁ 817.5 million (USD 1.553 billion, approximately) was determined, only
including direct costs.24 This is equivalent to ÂŁ 1,154
per person per year, around USD 2,300 per person per year.24 The average stay was 9.9 days.
Hospital expenses were 35% of the total to treat less than 2% of exacerbations.24
The mean calculation per hospitalization was ÂŁ 3,000 (USD 5,700),
as opposed to ÂŁ 100 (USD 190) for outpatient treatment.24 Probably this analysis
underestimates the real cost for the society, since it doesn’t consider
indirect costs (loss of producÂtivity, because COPD has 6 times more absence
from work than asthma), direct costs for the paÂtient (trips), or intangible
costs (quality of life). If we analyze the distribution of direct costs in the
United Kingdom, 47.5% was used for the purchase of drugs; 24.5% for home oxygen
therapy; 17.8% for hospital fees and 10.2% for medical outpatient fees.23, 24
With regard to COPD
hospitalization in ArgenÂtina, the National Institute of Respiratory DiseaÂses
“Dr. Emilio Coni” has informed that in 2015 almost 30,500 hospitalizations were
reported for exacerbated COPD in a sample including public institutions of the
country.13
In the GCBA, in 2013, there were 1,066 hospitalizations, and in
2014, 996 hospitalizations for exacerbated COPD.53
According to the
previous cost analysis in paÂtients with exacerbated COPD, twenty years ago,
including 33 patients, the total direct cost per hospitalized patient was USD
2,451. The structure of hospital costs was distributed as follows: costs of
final services represented 75% of total costs; 57% of those belonged to
salaries (17.55% for physicians, 37.41% for nurses and 1.51% for administrative
employees), and 13% to drugs, disposable maÂterials and medical practice
(drugs; 8.8% of the total amount).36 The remaining 25% was related
to the transfer of costs from general services and other services, with 12.48%
for the staff.36 The hospitalization day was
USD 163, the same as the cost per hospitalization day of the current analyÂsis
(USD 162), that is to say, it remained stable throughout two decades. It is
worth mentioning that oxygen therapy provided at the emergency department, the
regular ward and ICU represents a high hospital cost in all the cost analyses
of the disease. In our case, it is considered as part of the hospitalization
module, just like the use of invasive and non-invasive ventilation is
considered within the ICU module. At the time this study was being conducted,
the high-flow oxygen cannula wasn’t available.
Regarding the mean
hospitalization days of our current study, it has been reduced to 9 days, which
is a lot less than the average of this same institution twenty years ago (15
days). The average number of hospitalization days is similar to the one
reported internationally: 9.9 d in Great Britain and 7.75 d in the United
States.22-24, 36 One possible explanation could
be that the standardization of treatment and permanent training of healthcare
staff in our institution for the adequate and updaÂted management of patients
with COPD, as well as the availability of more effective pharmacological
treatments, have allowed a significant reduction in hospital length of stay.
Different international Guidelines (GOLD, ALAT, GESEPOC and others), plus the
Guidelines from the Ministry of Health of our country summarize the
recommendations for the effective management of COPD, with bronchoÂdilator
therapy as the cornerstone.2, 3, 54, 55
Both the
international and the national GuiÂdelines identify a group of patients who
share the fact of having been hospitalized the year before or more than one
outpatient exacerbation requiring antibiotics or systemic corticosteroids
(GROUPS C and D of the GOLD Guidelines). Given the worst prognosis of patients
who have suffered frequent exacerbations, with higher impairment of the
pulmonary function, symptoms, quality of life and mortality, the frequent
exacerbator phenotype is considered by all the Guidelines as the risk factor
with the worst prognosis, and deserves a more intensive treatment, even with
the use of inhaled corticosteroids.2, 3, 54-57 Other factors known as
hospitalization risks in COPD are: difficulty in accessing the pharmacological
treatment, nonadÂherence, inhalation errors and comorbidities.2,
3, 54, 55 In
our study, only 10% of patients had social seÂcurity. Also, the high prevalence
of mistakes in the administration of inhaled drugs in patients with chronic
obstructive diseases, such as asthma and COPD, is well-known around the world.58
Usmani et al determined that advanced age, low socioecoÂnomic
status and low educational level, the lack of previous training in the correct
inhalation therapy, and the presence of comorbidities were the factors
associated with the mistakes in the administraÂtion technique; all of this
related to bad asthma control and an increase in the use of healthcare
resources.59 With respect to comorbidities,
there was high prevalence in our study (Table 1), and it is known that they
have a bearing on the worst disease prognosis, both in the exacerbator
phenotype (groups C and D) and in the non-exacerbator group (Group B).2, 3, 54, 55, 60, 61
Regarding the
limitations of this study, we can say that data collection from the medical
records was retrospective. Another limitation is the fact that the
extrapolation of conclusions for other health systems in our country or other
regions (external validity) is not recommended, due to the different cost
structures, as we have already mentioned. Indirect costs haven’t been evaluated
(they are thought to be higher than direct costs, basing on previously reviewed
information), and costs haven’t been determined from other persÂpectives
(patient or society). Costs were initially calculated in pesos, but due to the
exchange rate instability and the devaluation suffered by our country in the
last years we decided to express the results in dollars. Finally, the
modulation used by the GCBA didn’t allow us to separate the internal cost
structure to know which variables have been considered, and to what extent.
To conclude, after
twenty years, a direct cost study of COPD exacerbation in hospitalized paÂtients
in a public hospital of CABA has been carried out again. The sample consists
mostly of men older than 60 years, with poor follow-up, high smoking load and
severe obstruction of the airflow. The direct cost from the funder’s
perspective was USD 1,462 per patient; the cost of a patient hospitalized at
the ICU was almost seven times higher. But we have to take into account that
only three patients were admitted to the ICU, and there was great disÂpersion
in the cost per patient. Most of the costs for ward hospitalizations had been
calculated in the module, but not in the case of the ICU, which uses high-cost
drugs. A significant reduction has been shown in hospitalization length of
stay, but with a day-bed cost that remained stable throughout 20 years (USD
162-163). Probably the indirect cost is much higher. We suggest the need to
include this type of study in the hospital environment for the purpose of
collecting data to allow a better management of the available resources.
Including cost-related problems in every affected area could contribute to the
management of available resouÂrces, the planning, organization and systematizaÂtion
of patient care, thus improving the production and quality of the end-product
with equal or lower budget. Being the management of COPD patients the most
important element of direct costs, systeÂmatic COPD management programs shall
be imÂplemented for the purpose of identifying patients with risk factors,
educating them about treatment adherence and allowing access to medication, in
order to reduce the number of hospitalizations for exacerbations and, why not,
the mortality.
Conflict of interest
Dr. Daniel Pascansky
has participated in continuous meÂdical education programs for GSK,
AstraZeneca, ELEA, Casasco and Novartis.
Dr. Martín
Sívori has participated in continuous meÂdical education programs for
GSK, AstraZeneca, TEVA and ELEA.
Dr. Luciano Capelli
has no conflict of interest to declare.
REFERENCES
1. Ball P, Make B. Acute
exacerbation of chronic bronchitis: An international comparison. Chest 1998;113:199S-204S.
https://doi.org/10.1378/chest.113.3_Supplement.199S
2. Global Strategy
for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary
Disease. NHLBI/WHO. Workshop
Report. 2023. Acceso en www.goldcopd.com. Consultado
el 14 Noviembre de 2022.
3. Figueroa Casas JC, Schiavi
E, Mazzei JA, et al. RecomendacioÂnes para la
prevención, diagnóstico y tratamiento de la EPOC en Argentina.
Medicina (B Aires) 2012;72 (Supl.I):1-33.
4. Cuarta Encuesta Nacional de Salud.
Ministerio de Salud. Argentina. 2018. Acceso en https://www.indec.gob.ar/ftp/cuadros/publicaciones/enfr_2018_resultados_definitivos.pdf. Consultado el 10 de
Marzo 2022
5. Sullivan S, Ramsey S, Lee T. The economic burden of COPD. Chest 2000;117:5S-9S.
https://doi.org/10.1378/chest.117.2_suppl.5S
6. Miravitlles M, Soriano JB, Garcia Rio F, et al. Prevalence of COPD in Spain: impact of undiagnosed COD on quialÂity of life and daily life activities (EPISCAN). Thorax 2009;64:863-8.
https://doi.org/10.1136/thx.2009.115725
7. Sobradillo Peña V, Miravitlles
M, Gabriel R, et al. GeoÂgraphic
Variations in Prevalence and Underdiagnosis of COPD:
Results of the IBERPOC Multicentre EpidemiologiÂcal
Study. Chest 2000;118:981-9. https://doi.org/10.1378/
chest.118.4.981
8. Menezes
AMB, Perez-Padilla R, Jardim JB, et al. Chronic obÂstructive
pulmonary disease in five Latin American cities (the PLATINO study): a
prevalence study. Lancet 2005;366:1875- 81.
https://doi.org/10.1016/S0140-6736(05)67632-5
9. Echazarreta AL, Arias SJ,
del Olmo R, et al. Prevalencia de EPOC en 6 aglomerados urbanos de Argentina:
el estudio EPOC.AR. Arch Bronconeumol
2018;54:260-9. https://doi.org/10.1016/j.arbres.2017.09.018
10. Schiavi E, Stirbulov R, Hernández Vecino R, Mercurio S, Di Boscio V, PUMA Team. COPD Screening in Primary Care in Four Latin American Countries:
Methodology of the PUMA Study. Arch Bronconeumol 2014;50:469-74. https://doi.org/10.1016/j.arbr.2014.09.010
11. World Health Organization. World Health Statistics 2022. Acceso
1 Octubre de 2022 en https://www.who.int/data/gho/
publications/world-health-statistics
12. Sivori M, Saenz C, Riva Posse C. Mortalidad
por Asma y EPOC en la Argentina de 1980 a 1998. Medicina (B Aires) 2001;61:513-21.
13. Bossio JC, Arias S.
Actualización de datos epidemiológicos sobre la EPOC. Instituto
Nacional de Epidemiología “Dr. Emilio Coni”.2020. (información
personal).
14. Mannino
DM, Buist AS. Global burden of COPD: risk facÂtors,
prevalence, and future trends. Lancet 2007;370:765-
73. https://doi.org/10.1016/S0140-6736(07)61380-4
15. Foster TS, Miller JD, Marton JP, Caloyeras JP, Russell
MW, Menzin J. Assessment of the economic burden of
COPD in the US: a reviews and synthesis of the literature. J COPD 2006;3:211-8. https://doi.org/10.1080/15412550601009396
16. Newhouse JP. Medical care
costs: how much welfare loss? J Econ
Persp 1992; 6: 3-21.
https://doi.org/10.1257/jep.6.3.3
17. Sculpher MJ, Pang FS, Manca A, et al. General disability in economic evaluation studies in healthcare: a
review and case studies. Health Technol Assess 2004;8:1-19. https://doi.org/10.3310/hta8490
18. Hilleman
DE, Dewan N, Malesker M,
Friedman M. PharmaÂcoeconomic evaluation of COPD.
Chest 2000;118:1278-85.
https://doi.org/10.1378/chest.118.5.1278
19. Starkie
JH, Briggs AH, Chambers MG. Pharmacoeconomics in COPD: lessons for the future.
Int J COPD 2008;3:71-8.
20. Del Negro R. Optimizing
economic outcomes in the manÂagement of COPD. Int J COPD 2008;3:1-10.
https://doi. org/10.2147/COPD.S671
21. Chapman KR. Epidemiology and
costs of chronic obstrucÂtive pulmonary disease. Eur Respir J 2006; 27: 188-207.
https://doi.org/10.1183/09031936.06.00024505
22. Sharafkhaneh
A, Petersen NJ, Yu HJ, Dalal AA, Johmson
Ml, Hanania NA. Burden of COPD in a
government health care system. Int J COPD 2010;6:125-32.
23. Guest J. Assessing the cost
of illness of emphysema. Dis Manage Health Outcomes 1998;3:81-8.
https://doi. org/10.2165/00115677-199803020-00004
24. Guest J. The
annual cost of chronic obstructive pulmoÂnary disease to the UK´s National
Health Service. Dis Manage Health Outcomes 1999;5:93-100.
https://doi.org/10.2165/00115677-199905020-00004
25. Miravitlles
M, Murio C, Guerrero T, Gisbert
R. Costs of chronic bronchitis and COPD: a 1-year follow-up study. Chest 2003;123:784-91. https://doi.org/10.1378/ chest.123.3.784
26. Jansson
S, Andersson F, Borg S, et al. Costs of COPD in
Sweden according to disease severity. Chest 2002;122:1994-
2002. https://doi.org/10.1378/chest.122.6.1994
27. Pelletier-Fleury
N, Lanoe JL, Fleury B, Fardeau M. The cost of treating COPD
patients with long-term oxygen therapy in a French population. Chest
1996;110:411-6. https://doi.org/10.1378/chest.110.2.411
28. Nielsen R, Johannssen A, Bendiktsdottir B,
et al. The economic burden of COPD in a US Medicare
population. Respir Med 2008;102:1248-56.
https://doi.org/10.1016/j.rmed.2008.04.009
29. Akazawa
M, Halpern R, Riedel A, et al. Economic burden prior to COPD diagnosis: a
matched case-control study in United States. Respir
Med 2008;102:1744-52. https://doi.org/10.1016/j.rmed.2008.07.009
30. Miravitles
M, Broisa M, Velasco M, et al. An
economic analysis of pharmacological treatment of COPD in Spain. Respir Med 2009;103:714-21.
https://doi.org/10.1016/j.rmed.2008.11.019
31. Nielsen R, Johannssen A, Bendiktsdottir B,
et al. Present and future costs of COPD in Iceland and Norway: results from the
BOLD study. Eur Respir J 2009;34:850-7. https://doi.org/10.1183/09031936.00166108
32. Del Negro RW, Tognella S, Tosatto R, et al.. Costs of COPD in Italy: the SIRIO study (social impact of
respiratory inÂtegrated outcomes). Respir Med 2008;102:92-101. https://doi.org/10.1016/j.rmed.2007.08.001
33. Izquierdo
Alonso JL, de Miguel Diez J. Economic impact of
pulmonary drugs on direct costs on stable COPD. J COPD 2004;1:215-23.
https://doi.org/10.1081/COPD-120039809
34. Miller JD, Foster T,
Boulanger L, et al. Direct costs of COPD in the US: an analysis of medical expenditure
panel survey (MEPS) data. J COPD 2005;2:311-8.
https://doi.org/10.1080/15412550500218221
35. Gerdtham
UG, Andersson LF, Ericcson
A, et al. Factors affecting COPD-related costs: a multivariate analysis of a
Swedish COPD cohort. Eur J Health Econ 2009;10:217-26. https://doi.org/10.1007/s10198-008-0121-6
36. Saénz C, Sivori M, Blaho E, Sanfeliz N. Costos en la EPOC: Experiencia en el Hospital Dr.J.M.Ramos Mejia y
revisión de la literatura. Rev Arg Med Respir
2001:1:45-51.
37. Bakerly
ND. Cost analysis of an integrated care model in the management of acute
exacerbations of COPD. Chronic Respir Dis 2009;6:201-8. https://doi.org/10.1177/1479972309104279
38. Effing T, Kestejens
H, Van der Valk P, et al. Cost-effecÂtiveness of
self-treatment of exacerbations on the severity of exacerbations in patients
with COPD: the COPE II study. Thorax 2009;645:956-62.
https://doi.org/10.1136/ thx.2008.112243
39. Steuten
L, Lemmens K, Nieboer A, Vrijhoef H. IdentifiyÂing
potentially cost-effective chronic care programs for people with COPD. Int J COPD
2009;4:87-100. https://doi.org/10.2147/COPD.S3047
40. Puig Junoy J, Casas A,
Font-Planells J, et al. The impact of home hospitalization on healthcare costs
of exacerbations in COPD patients. Eur J Health Econ
2007; 8:325-32. https://doi.org/10.1007/s10198-006-0029-y
41. Schermer
TR, Saris CD, van den Bosch WJ, et al. ExacerbaÂtions and associated healthcare
cost in patients with COPD in general practice. Monaldi
Arch Chest Dis 2006;65:133- 40. https://doi.org/10.4081/monaldi.2006.558
42. Simoni-Wastila
L, Yang HW, Blancehtte CM, et al. HosÂpital and
emergency department utilization associated with treatment for COPD in a managed care Medicare utilization. Curr
Med Res Opin 2009;25:2729-35.
https:// doi.org/10.1185/03007990903267157
43. Simoens
S, Decramer M. Pharmacoeconomics
of the manageÂment of acute exacerbations of COPD. Exp
Pon PharmacothÂer 2007;8:633-48. https://doi.org/10.1517/14656566.8.5.633
44. Guarascio
AJ,Ray SM, Finch CK, Self
TH. The clinical and economic burden of COPD in USA. ClinEcon Outcomes Res 2013;5:235-45
45. Nomenclador del Ministerio de Salud del Gobierno de
la Ciudad Autónoma de Buenos Aires. Datos Personales. Junio 2021.
46. Manual Farmacéutico Kairos.
Junio 2021
47. Afolabi
AO, Watson B, Procter S, Wright AJ. The Cost to the Health
Service of Chronic Obstructive Pulmonary Disease. Eur Resp
J 2000;16,31S:13.
48. Strassels
S, Smith D, Sullivan S, Mahajan P. The
costs of treating COPD in the United State. Chest 2001;119:334-52.
https://doi.org/10.1378/chest.119.2.344
49. Murray CJ, Atkinson C, Bhalla K, et al. The state of US health,1990-2010:
burden of diseases, injuries and risk factors. JAMA 2013;310:591-608. https://doi.org/10.1378/
chest.119.2.344
50. Trapero Bertran M, Oliva
Moreno J, y Grupos de Expertos GECA. Guía metodológica para la
estimación de los costes en asma. Luzan 5, SA
de Ediciones.2017.
51. Bilde
L, Svenning R, Dollerup J, Bække Borgeskov J, Lange P.
The cost of treating patients with COPD in Denmark - A population study of COPD
patients compared with non- COPD controls. Respir Med
1999;101:539-46. https://doi.
org/10.1016/j.rmed.2006.06.020
52. Hoogendoorn
M., Rutten-van Mölken
MP., Hoogenveen RT et al. A dynamic population model
of disease progression in COPD. Eur Respir J 2005;26:223-33. https://doi.org/10.1183/09031936.05.00122004
53. Dirección de Estadísticas en Salud del
Ministerio de Salud del Gobierno de la Ciudad Autónoma de Buenos Aires.
2021.
54. Montes de Oca M, Lopez
Varela V, Acuña A, et al. Guía de práctica clínica
de la EPOC ALAT 2014: Preguntas y respuestas. Arch Bronconeumol 2015;51:403-16.
https://doi.org/10.1016/j.arbres.2014.11.017
55. Miravitlles M, Soler
Cataluña JJ, Calle M, et al Guía español de la EPOC (GesEPOC) 2017. Arch BroncoÂneumol 2017;53:324-335.
https://doi.org/10.1016/j.arÂbres.2017.03.018
56. Vestbo
J, Anderson W, Coxson HO, et al. Evaluation of COPD
longitudinally to identify predictive surrogate end-points (ECLIPSE). Eur Respir J 2008;31:869–73. https://doi.org/10.1183/09031936.00111707
57. Soler-Cataluña
JJ, Martínez-García MA, Román Sánchez P, Salcedo
E, Navarro M, Ochando R. Severe acute exacerÂbations
and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005;60:925-31.
https://doi. org/10.1136/thx.2005.040527
58. Sivori M, Balanzat A, Casas JP, et al. Inhaloterapia:
RecoÂmendaciones para Argentina 2021. Medicina Buenos Aires 2021;81 (Supl II):1-32.
59. Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of
impact on health outcomes. Respir Res 2018;19: 10. https://doi.org/10.1186/s12931-017-0710-y
60. Sivori M, Fernández
R, Toibaro J,Velasquez
Gortaire E. Supervivencia en una cohorte de pacientes
con EPOC acorde a la clasificación GOLD 2017. Medicina Buenos Aires 2019;79:20-8.
61. Jimenez J, Sivori M. Evaluación de las comorbilidades por los
índices de Charlson y COTE en la EPOC y su
relación con la mortalidad. Revista Americana de Medicina RespiÂratoria
2022;22:3-9.