Autor : Dra. Nadia M. Figueroa1 Dr. Alberto A. Marangoni1
1 Sanatorio Allende. Servicio de Diagnóstico por Imágenes. Córdoba. Argentina
Correspondencia :finami15@gmail.com. Nadia M. Figueroa
Abstract
Introduction: Inspired by the BI-RADS system (Breast Imaging Reporting and Data
System), the American College of Radiology (ACR) developed the Lung-RADS
system, for the purpose of making standardized reports on lung nodules detected
in lung cancer screening (LCS). In Argentina and in many other parts of the
world the LCS is not performed due to high costs; however, in chest CT scans
pulmonary nodules frequently appear as incidental findings. There are multiple
systems to evaluate them based on a series of features that allow subsequent
follow-up. Some of them are the Fleischner Guidelines, the British Thoracic
Society Guidelines and the Lung-RADS system, the latter being the only one with
numerical categorization. In this article we study the usefulness of the Lung-
RADS, as a diagnostic, follow-up method for the classification of pulmonary
nodules. Objective: Evaluation of the pulmonary nodule diagnosed on chest CT
scan, using the Lung-RADS system to determine its clinical importance,
comparing the correlation between this classification and the malignancy or
benignancy in the histopathological examination.
Material and Method: Descriptive, statistical, observational, retrospective and prospective
study. A total of 100 adult patients, both men and women, with a diagnosis of
pulmonary nodule were studied between January 2017 and December 2019. Patients
without follow-up were excluded. Studies were performed with a 128-slice
scanner. The variables under evaluation were: patients’ sex and age, size and
density of the nodule, malignancy of the lesion found in the
anatomopathological study, Lung-RADS category and treatment performed and
suggested. For the descriptive analysis we used relative frequencies
(percentages) and absolute frequencies (number of cases) for qualitative
variables; and mean and standard deviation as well as range of minimum-maximum
values for the quantitative variables. For hypothesis tests, Chi-Square tests
were performed for qualitative variables. For quantitative variables, Shapiro
Wilks and Kolmogorov tests were performed.
Results: In 100 patients in whom Lung-RADS was applied to determine follow-up and
treatment, different types of scenarios could be identified regarding the approach
and follow-up: some needed recategorization and changes in the diagnostic
approach and treatment. As for the statistical analysis, we analyzed the
association between the Lung-RADS classification obtained and the presence or
absence of malignancy in the anatomopathological examination, and obtained
statistically significant results (p-value <0.0001) for this association.
Discussion: The Lung-RADS system and the Fleischner Society Guidelines on pulmonary
nodules are used at present. Both have similar criteria and are based on the
morphological suspicion of malignancy that includes the density of the nodule
(solid, partially solid or ground-glass), the size and, when available, growth
or evolution, which can be applied in different groups of patients. Determining
the Lung-RADS score has proven its usefulness in this study, based on the
pathological correlation of the nodule, with a statistically acceptable result
and a good correlation with the treatment and follow-up decision.
Conclusion: The application of the Lung-RADS system to this series of patients has
shown a good management of patients’ follow-up, with surgical resections in
some cases and an expectant approach in others, providing certain security and
mostly avoiding the use of unnecessary aggressive treatments.
Key words: Nodule, Lung-RADS, CT, Approach
Received: 01/13/21
Accepted: 07/09/2021
Introduction
Inspired by the BI-RADS system
(Breast Imaging Reporting and Data System), the American College of Radiology
(ACR) developed the Lung-RADS system, for the purpose of making standardized
reports on lung nodules detected in patients who had undergone lung cancer
screening (LCS), thus reducing the risk of overdiagnosis or unnecessary
surgeries, taking into account the fact that lung cancer is one of the leading
causes of cancer death in men and women at present.1 That is why we must emphasize
the importance of early detection of lung cancer, with timely follow-up of the
pulmonary nodules, whether in detection programs (carried out in developed
countries) or in cases of incidental findings in countries where LCS is not
performed.
As a matter of fact, LCS is not
performed in Argentina yet, but patients and health staff can use the chest CT,
with frequent incidental findings of pulmonary nodules. For those cases,
several guidelines are used, such as the Fleischner Society and the British
Thoracic Society Guidelines, which share treatment and follow-up criteria with
the Lung-RADS system, the latter being the only one with a numerical
classification scale.
Taking into account the available
guidelines, this study evaluates the usefulness of the Lung-RADS system (Figure
N° 1) using it as a follow-up method (considering the fact that it provides
the numeriÂcal value based on imaging findings) for the classification of pulmonary
nodules in order to apply a follow-up scheme for patients with these nodules.
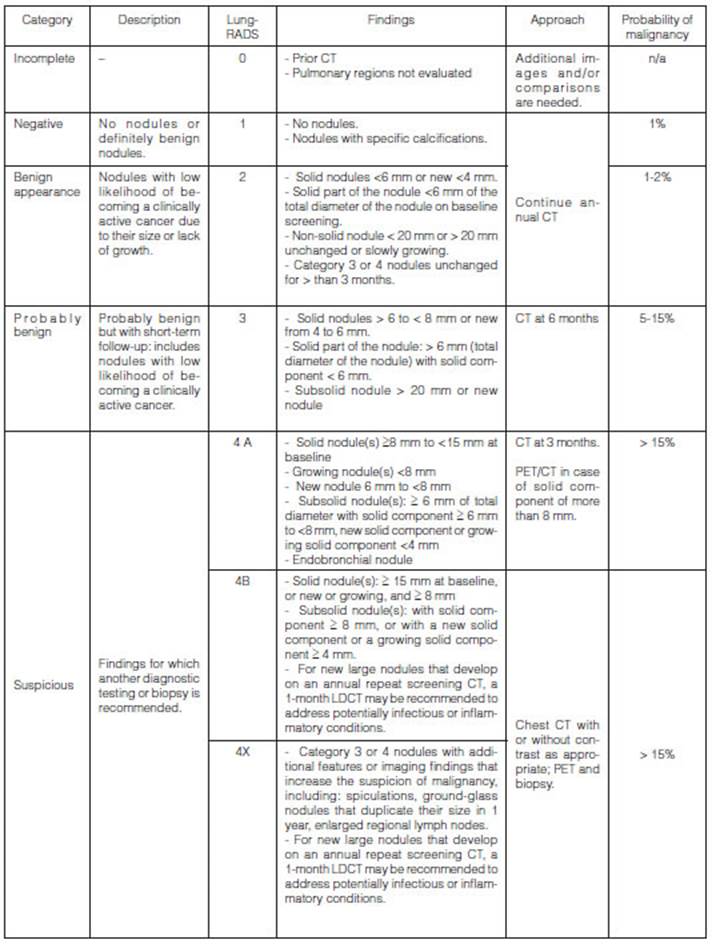
Based on this system and the
recommendations of the general literature, this study retrospectively and
prospectively evaluates patients with solitary pulmonary nodule (SPN) applying
the Lung-RADS system to determine if this nodule classification can establish a
specific expectant or surgical approach to consider its real value.
Objective
To determine the clinical value
of the Lung-RADS system in the study of the solitary pulmonary nodule for determining
the probability of malignancy of such nodule.
Study hypothesis
The use of the Lung-RADS system
for the analysis of pulmonary nodules determines the probability of malignancy
of the lesion and provides data useful for considering which approach to be
used: expectant, active monitoring or therapeutic.
Materials and method
Descriptive, statistical,
observational, retrospective and prospective study.
The American College of Radiology
(ACR) developed the Lung-RADS using the computed tomograÂphy (CT) for lung
cancer screening (LCS) with established criteria that depend on different
organizaÂtions, such as the ACS (American Cancer Society), ACCP (American
College of Chest Physicians), ALA (American Lung Association), ASCO (American
Society of Clinical Oncology), ATS (American Thoracic Society) and Centers for
Medicare and Medicaid Services, with factors in common: age (≥ 55 years)
and smoking history (if the patient is a current smoker, ≥ 30 packs/year,
or if the patient quit smoking 15 years before or less).
Inclusion criteria: we selected
100 adult patients diagnosed with solitary pulmonary nodule detected by chest
CT, taken from the database of the Diagnostic Imaging Service.
Exclusion criteria: pediatric
patients, patients with Lung-RADS 0 and 1 and also patients with known
neoplasia.
For the chest
computed tomography we used a GE® Optima 660 128-slice scanner.
The study was performed interchangeably with or without contrast injection
using multi-slice volumetric acquisition and reconstruction of 0.63 mm of
thickness, with 5 mm slice distance in axial, sagittal and coronal planes for
the high-resolution system, with inspiratory apnea and completing with
discontinuous slices in sustained espiration, and for conventional acquisition,
a thickness of 3.75/4 mm every 4 mm. Also, the densitometric MIP (maximum
intensity projection) and MinIP (minimum intensity projection) reconstructions
were performed and analyzed. The settings of the CT studies were: helical
scanning; tube rotation speed, 0.6 seconds; full scan length at 120 Kv; from
100 to 500 mAs automatically set; 1.375:1 of pitch; 40 mm of detector coverage;
a percentage of automatic and an average DLP (dose length product) of 900 to
1300 mGy (depending on thickness and height of the patient, due to automatic
dosage of the equipment), with a scan time of 9 seconds for the sequence. For the
low-dose studies, 120 Kv and 18 to 200 mAs were used (percentage of dose
reduction between 40 and 50%), with a scan time of 7 seconds and determining an
average DLP of 500 to 700 mGy. (DLP: [mGy ∗ cm] = CTDIvol [mGy] ∗ Scanner length [14 cm]).
For the
histopathological examination, staining with hematoxylin and eosin. For patients that needed
immunostaining we used specific antibodies for each tumor lineage, depending on
the result obtained in the histopathological examination performed in the first
instance.
Surgeries recommended
for the treatment were mostly lobectomies and the
surgical technique used was conventional or video-assisted, depending on the
requirements of each particular case.
Diagnostic procedure: data were collected from the records of patients who had undergone
chest CT since 2017. Chest CTs were reviewed, and those which showed pulmonary
nodules were evaluated, measuring the diameter of the nodules in the lung
window (following the current recommendations of the Fleischner Guidelines),
obtaining an average diameter between the long and short-axis diameters,
including decimals. In cases of multiple nodules, only the most suspicious one
was to be measured. A nodule was considered to have grown if it had an increase
in size of ≥ 1.5 mm.
Patients with Lung-RADS 1
(including granulomas and hamartomas) were excluded as well as those with known
neoplastic processes.
The study variables were:
patients’ sex and age, size and density of the nodule, malignancy of the lesion
found in the anatomopathological study, Lung-RADS category and treatment
performed and suggested, expectant or surgical approach and determining the
stage of the disease at the moment of the diagnosis. It was considered as
advanced stage when local infiltration, infiltration of neighbor structures
(pleura, pericardium, central bronchi), heart and great vessels, bone; and
extranodal extenÂsion (metastasis in extra-pulmonary organs or in the contralateral
lung) could be identified.
Patients’ data were collected
retrospectively in order to allow for a more precise and effective study, apart
from the patients who were evaluated prospectively from January 2018. In this
regard, we must emphasize the fact that LCS is not performed in Argentina. So,
categorized pulmonary nodules were analyzed basing on the findings obtained
from imaging in relation to an incidental finding or pulmonary study for
clinical symptoms and not through screening, thus smoking wasn’t taken into
account as a variable, since the objective was to assess mainly the score and
no additional data.
Some of the patients evaluated in
a prospective manner needed Lung RADS recategorization during follow-up. For
the statistical analysis we used the last recategorization. The description of
those cases is specified later.
On the other hand, patients who
remained stable after periodic follow-ups were recategorized to Lung-RADS 2,
and so continued with an annual follow-up without signs of malignancy to date.
Categorized pulmonary nodules
were analyzed based on the findings seen in the chest CT in all adult patients,
with no age or sex distinction and not differentiating between smokers and
non-smokers, but including patients older than 18 years. The Lung-RADS system
was used for the analysis, categorization and selection of follow-up approaches
for patients with CT-detected pulmonary nodules.
Statistical analysis:
for the descriptive analysis, we used relative
frequencies (percentages) and absolute frequencies (number of cases) for
qualitative variables; and mean and standard deviation as well as range of
minimum-maximum values for the quantitative variables. For hypothesis tests,
Chi- Square tests were performed for qualitative variables. For quantitative
variables, Shapiro Wilks and Kolmogorov tests were performed in the first place
in order to analyze variable distribution. Then, Wilcoxon tests were carried
out to observe differences between groups. A value of p <0.05 was considÂered
to be statistically significant.
The software used for data
analysis was Excel and Infostat professional version 2019.
Results
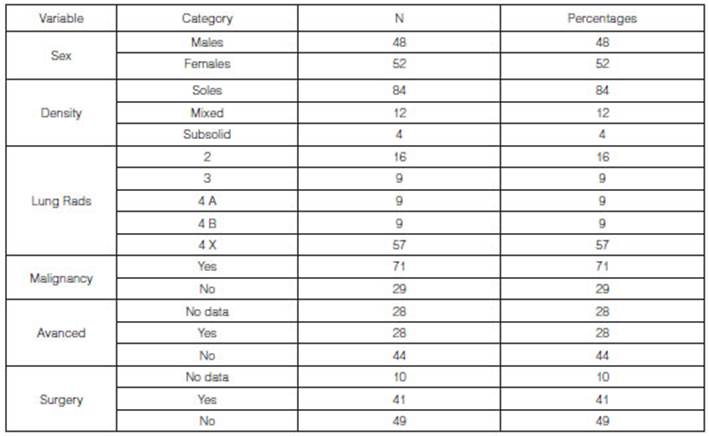
Data were analyzed from 100
patients, mean age 60 (± 14 years) and an age group between 22 and 90 years.
Regarding sex, there were 52 males (52%) and 48 females (48%).
The average size of the nodules
was 23.97 ± 16.61 mm, with a minimum diameter of 5 mm and a maximum of 80 mm.
From the total number of cases of
the study (n = 100) we found 84 cases (84%) of patients with solid tumors. 57%
of the total number of patients (n = 57) had a Lung-RADS classification of 4X
and 71% of the population being studied showed malignant tumors (n = 71). With
regard to the staging of the tumors, 44% of the total number of patients didn’t
show an advanced stage (n = 44). 41 patients underwent surgery.
The analysis between the approach
suggested by the Lung-RADS system and the final approach used for the diagnosis
and treatment of the whole sample (n = 100) has shown that in 66% of the cases
(n = 66), the Lung-RADS approach indicated a biopsy, a PET study (positron
emission tomography) or surgery, whereas in 16% of the cases (n=16) it
suggested annual check-up. The analysis of the selected approach used in all
the patients showed that a biopsy was requested in 34% of the cases (n=34) and
28% (n = 28) were requested to undergo surgery.
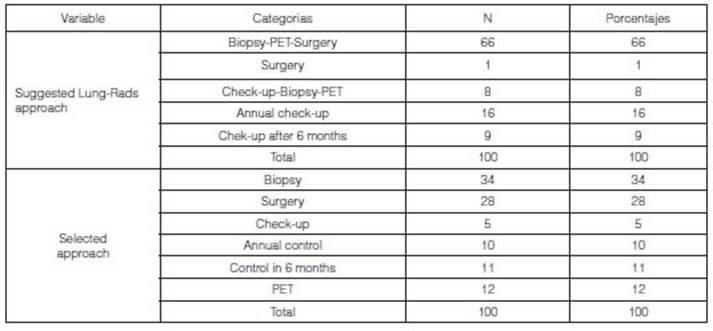
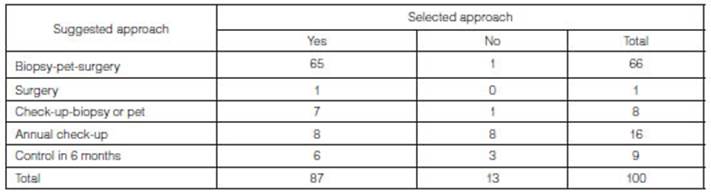
Based on the approach that was
chosen, we analyzed if it corresponded with the one suggested by the Lung-RADS
(Table 3). 87 of the 100 patients underwent the treatment suggested by
the Lung-RADS (87%). Results were statistically significant (p-value
<0.001).
Then, we analyzed the association
between the Lung-RADS classification obtained and the presence or absence of
malignancy in the anatomopathological examination (Graphic 1).
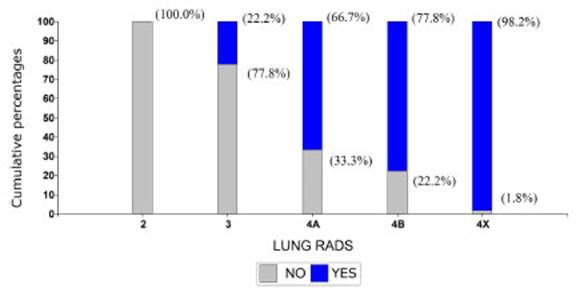
100% of the cases classified as
Lung-RADS 2 were free from malignancies, but this percentage started to
decrease as the Lung-RADS classification score got higher: among patients with
Lung-RADS 3, only 22.2% showed malignancy; in Lung-RADS 4A the percentage
increased to 66.7%; and in patients with Lung-RADS 4B, it increased to 77.8%.
98.2% of patients with Lung-RADS 4X showed malignancy. The results of this
association were statistically significant (p-value<0.0001).
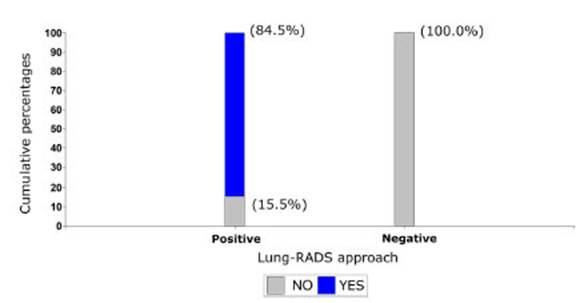
For a better statistical
analysis, the Lung-RADS classification was reduced to two groups: positive
cases and negative cases. Cases considered as negative were patients with
Lung-RADS 2 (n=16); and positive cases were patients with Lung-RADS 3 and 4
(n=84). With such grouping, we analyzed the association with the malignancy of
the tumor (Graphic 2) and observed that none of the patients with
negative Lung-RADS showed malignant tumors in the histopathology. In 15.5%
(n=13) of patients with positive Lung-RADS, tumors weren’t malignant. This
association was statistically significant (p-value <0.0001).
On the basis of this analysis, we
calculated the predictive values of the study and its sensitivity/ specificity.
The positive predictive value (PPV) of the population being evaluated was 100%,
that is to say, all the subjects who had a malignant result in the
histopathological examination were categorized as Lung-RADS 3 or 4.
The negative predictive value
(NPV) of the population being evaluated was 55.2%, meaning that all the
patients who had a benign result in the histopathological examination (100%:
n=29), the 55.2% (n=16), were categorized as Lung-RADS 2.
The sensitivity of the study was
84.6%, and the specificity, 100%. So, by doing these studies, the probÂability
of a Lung-RADS 3 or 4 nodule to have a positive result (malignant tumor) is
84.6%, whereas the possibility of a Lung-RADS 2 nodule to have a negative
result (benign tumor) was 100%.
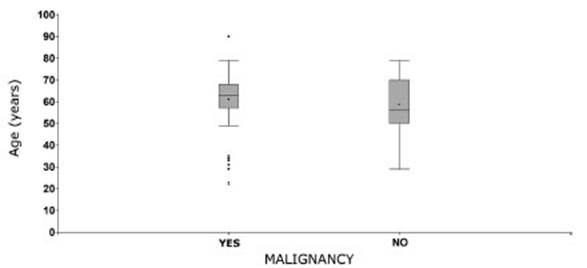
We also analyzed whether the
patients’ age was related to the malignancy of the tumor (Graphic 3). In
this case, the mean age observed in each group was similar and the differences
weren’t statistically significant (p-value: 0.1963). The mean age in the group
of patients with malignancy was 61 ± 14 years, whereas the group without
malignancy had a mean age of 59 ± 13 years.
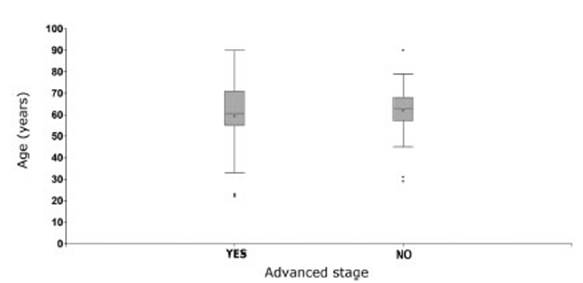
The same analysis was carried out
to observe if there was any relationship between the stage of the tumor and the
age of the patient. Patients with advanced stage had a mean age of 59 ± 17
years, and those without an advanced stage had a mean age of 62 ± 12 years. The
differences observed weren’t statistically significant (p-value: 0.7374) (Graphic
4).
In patients with malignant
tumors, we analyzed if the stage of the tumor, whether it was at an adÂvanced
or early stage could be related to the classification obtained with the
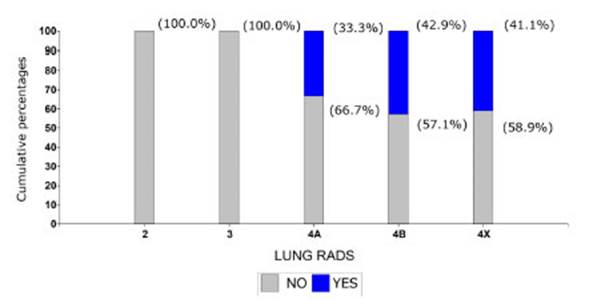
Lung-RADS (Graphic 5). In patients with Lung-RADS 3 classification there
weren’t any cases of advanced stage tumors. Among patients with Lung-RADS 4A
there were two advanced-stage cases (which accounted for 33.3% of the patients
of that group). Among Lung-RADS 4B and 4X patients, the percentages of advanced
cases were similar: 42.9% and 41.1%, respectively. Results weren’t
statistically significant (p-value: 0.7090).
Finally, we evaluated if there
was any association between the stage of the tumor and the classificaÂtion
obtained with the Lung-RADS system in patients with malignant tumors, according
to the density shown in the studies. In patients with malignant tumors, we only
observed cases of solid and mixed density (Table 4).
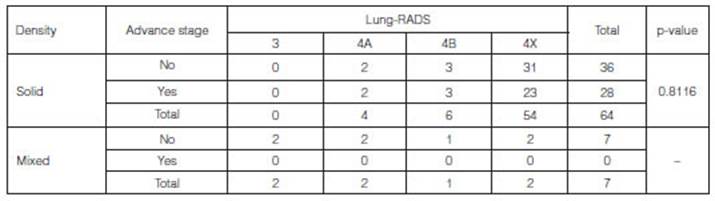
None of the patients with mixed
density (n=7) showed advanced stages.
In patients with solid density,
the differences observed between advanced and early stage weren’t statistically
significant (p-value: 0.8116).
Patients with Lung-RADS 4A with
advanced-stage tumors represented 50% of that group (n=2), whereas in patients
with Lung-RADS 4B and 4X, the percentage was 50% (n=3) and 42.6% (n=23),
respectively.
In the descriptive study we
observed cases in which after monitoring it was necessary to recategorize the
Lung-RADS and thus change the diagnostic/therapeutic approach.
One of the patients of the
Lung-RADS 2 category showed recategorization to 4A for an increase in size and
change in the density of the lesion, followed by surgery and diagnosis of
pulmonary adenocarÂcinoma. Then continued with follow-ups with annual
tomographic studies.
Another patient with a pulmonary
nodule also categorized as Lung RADS 2 in the first instance showed a subsolid
nodule that remained stable for 4 years (in this case a review was carried out
of previous tomographic studies), identifying certain growth after that time.
It was treated with surgery and the definitive diagnosis was lepidic growth
adenocarcinoma.
On the other hand, some patients
were categorized as Lung-RADS 3 in the first instance and then recategorized as
Lung-RADS 4 due to the growth of the lesion, observed in 2 patients, with
subsequent surgery and diagnosis of lung cancer in both cases.
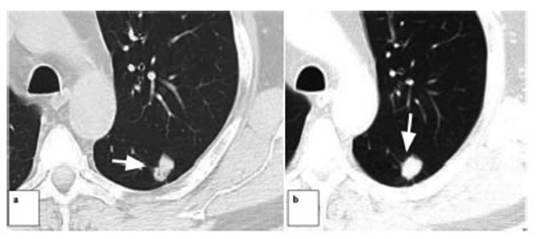
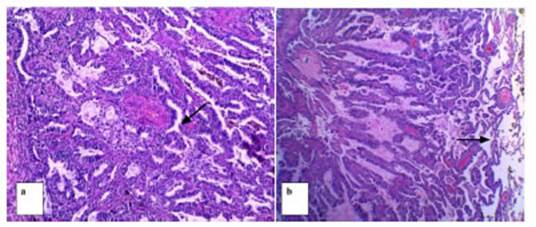
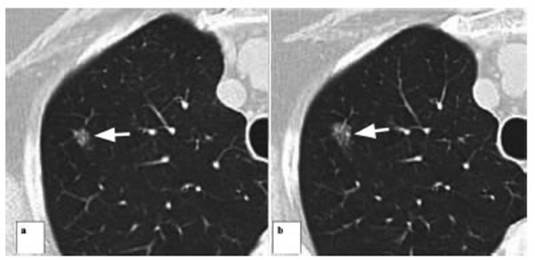
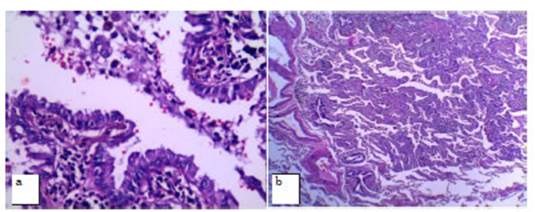
One of the patients who presented
a subsolid nodule with a central solid component of 6 mm in diameter
categorized as Lung-RADS 4A underwent a percutaneous biopsy under tomographic
guide, with a histopathological diagnosis of AAH (atypical adenomatous
hyperplasia). Follow-up done 6 months later showed that the lesion was stable;
continued with periodic follow-ups by CT, and recategorized as Lung RADS 3.
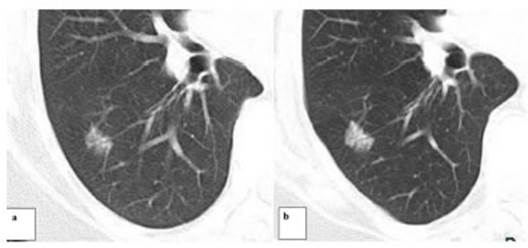
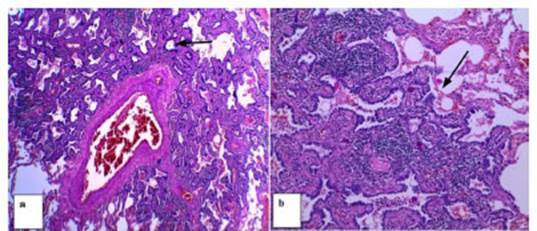
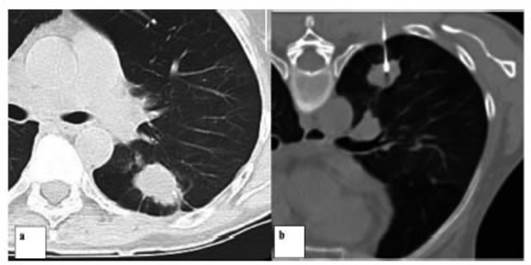
Another patient categorized as
Lung RADS 4X underwent a percutaneous biopsy under tomographic guide and was diagnosed
with hypersensitivity granulomatous alveolitis.
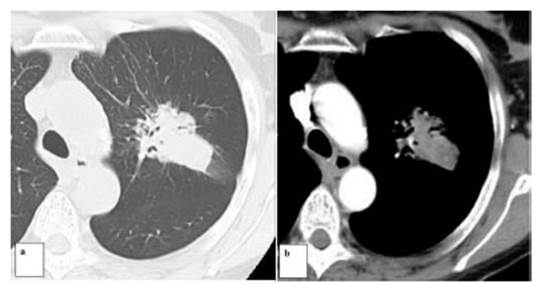
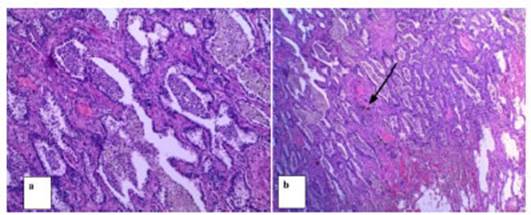
Discussion
The evaluation of pulmonary
nodules has significantly developed in the last years for the purpose of
reducing morbidity and mortality, looking for the timely and early detection of
lung cancer and findÂing the most suitable treatment for each particular case.3
For some time the characteristics of these nodules have been
evaluated in search for answers to achieve the optimum management of the pulÂmonary
nodule,4
taking into account the size, borders and usefulness of specific
methods to that end. The first important breakthrough on this subject was made
by the NTLS (National Lung Screening Trial), the first randomized multicenter
study to compare lung cancer screening in chest X-ray with low-dose computed
tomography, showing the possibility of detecting lung cancer at early stages,4
with good survival in patients with surgical resection (5-year
survival rate of 70%). After this study, several scales were created to
classify pulmonary nodules and avoid excess of treatments and invasive proceÂdures
and radiation therapy. At present, the most widely used methods for the
follow-up of pulmonary nodules are the Lung-RADS system and the recommendations
on pulmonary nodules of the Fleischner Society and the British Thoracic Society
guidelines5.
All of them share similar criteria and are based on the morphologic suspicion
of malignancy that includes the density of the nodule (solid, partially solid
or with ground-glass), the size and, when available, growth or evolution, which
can be applied in different groups of patients.
On the other hand, these
recommendations clarify the situation helping the specialist provide specific
and precise information to the patient, also reducing negative psychosocial
factors6.
In this regard, we must explain
that, despite the fact that smoking is an important underlying factor in the
detection of lung cancer, it is not included in this study because its
objective was to assess the score with no additional data.
Also, the different guidelines
share some practical points for a more specific classification. One of them,
the fact that the nodule shall be measured in the lung window, with 1.5 mm
thick cuts and obÂtaining an average size if there is irregular morphology7.
The Lung-RADS system8
is currently applicable to lung cancer screening; it follows the
classificaÂtion method of breast cancer and turns continuous data into categorized
information according to the systematic grading of the nodules by 4 basic
categories determined by the morphologic suspicion of malignancy, taking into
account the most suspicious nodule if there is more than one9.
Also, the Lung-RADS system has recommendation guidelines for the follow-up of
particular cases where paÂtients cannot be categorized specifically for having
ambiguous scenarios10;
however, those scenarios weren’t suggested in the cases included in this study
because these guidelines have been used outside a screening program.
In Argentina there isn’t any real
published information about screening for early detection of nodules and it
isn’t usually used in routine studies. Nevertheless, given the very high
incidence and accidental detection of pulmonary nodules in routine exams, the
Lung-RADS system was applied to the patients of this series for the study of
pulmonary nodules discovered by other causes or as a conÂsequence of individual
search of tumor disease, because since it has a numerical scale, it provides a
more practical classification compared to other available guidelines with the
same objective, allowing for better dialog and understanding between the
different specialists involved in the management of pulmonary nodules.
According to what is established
in the guidelines, small solid nodules, or nodules associated with a lepidic
component, or with central or popcorn calcifications (Lung-RADS 1) are usually
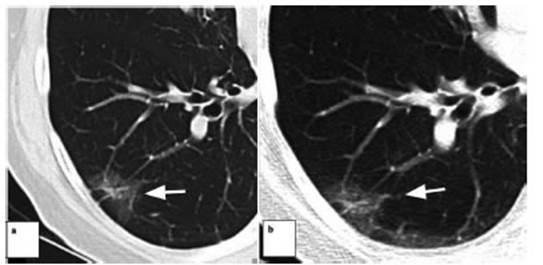
hamartoÂmas or granulomas, and remain stable. In this study, patients
categorized with Lung RADS 1 were excluded, but we included those with Lung
RADS 2, that is to say, small nodules (more than 4 mm). Among the nodules that
were evaluated in this series of patients (categorized as Lung-RADS 2), there
was one exception due to the need to recategorize and the final malignancy
finding; a percutaneous needle biopsy was performed, followed by surgery,
considering the growth and morphologic change of the nodule, with
adenocarcinoma as final diagnosis. So, despite the initial clinically low-risk
staging, it is necessary and very important to do the follow-ups indicated by
currently available guidelines within the suggested time period, in the event
of a Lung-RADS 2 false negative.
In fact, many patients needed to
be recategorized during subsequent check-ups. A study done in the National Lung
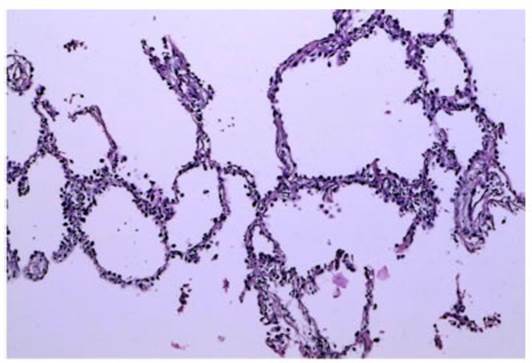
Cancer Center Hospital in Tokyo, Japan, explains that subsolid nodules of less
than 5 mm and with pure ground-glass pattern are mostly lesions of atypical
adenomatous hyperÂplasia (AAH). According to the anatomopathological
guidelines, they represent a precursor lesion of adenocarcinoma, because they
are atypical proliferations of less than 0.5 mm of cube-shaped cells throughout
the alveoli and in a large number of cases they have been observed in
association with pieces of adenocarcinoma.12 Some of those lesions may show
an increase in their size or may develop a solid component after 3 to 5 years
approximately; that is why some guidelines do not recommend annual follow-up4.
Another study conducted in Tokyo
also associated the development of AAH with a genetic predisÂposition and has
proven its coexistence with malignant lung lesions, both primary and secondary,
also stating that, despite the fact that smoking doesn’t play a part in its
appearance, it does in its transformation and evolution towards a neoplastic
lesion13.
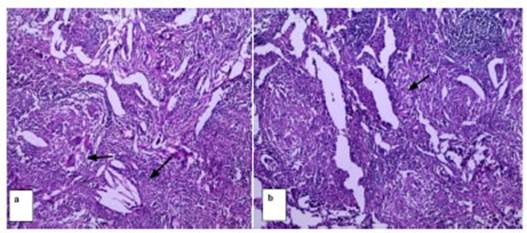
The findings of this study
correlate with this data. Follow-up was administered for 4 years in 2 patients,
and an increase in the size of the nodule was seen in the last check-up,
resulting in the recategorization of the Lung-RADS and the decision to perform
surgical resection. As an anatomoÂpathological result, the diagnosis was
early-stage lepidic adenocarcinoma.
In our statistical analysis, we
studied the association between the Lung-RADS classification obÂtained and the
presence or absence of malignancy in the anatomopathological examination (Figure
2). 100% of the cases classified as Lung-RADS 2, currently receiving annual
follow-ups, were free from malignancies. But this percentage started to
decrease as the Lung-RADS classification score got higher. Among patients with
Lung-RADS 3, only 22% showed malignancy; in Lung-RADS 4A this percentage
increased to 67%; and in patients with Lung-RADS 4B it increased to 77%. 98% of
patients with Lung-RADS 4X showed malignancy. The results of these associations
between the tomographic/ clinical categorization and histopathology were
statistically significant (p-value <0.0001).
Likewise, an article published in
2016 by the journal of the American College of Radiology showed that the ACR
application, Lung-RADS, increased the positive predictive value in a cohort of
CT lung screening by a factor of 2.5, at 17.3%, without increasing the number
of tests with false negative results15.
It is possible that the
insufficient number of patients is a weakness of this work, but we can deduce
that the use of a categorization system such as Lung-RADS is crucially
important for the follow-up of pulmonary nodules, considering that patients who
underwent tomographic check-ups according to the corresponding category and
showed visible changes in the tomography were benefited from early diagnosis
with good survival; and patients with early stage nodules with ground-glass or
mixed component remained stable and showed no signs of progression when data
were collected. (n = 12).
Patients diagnosed with early
stages underwent surgery. Only 27% of the patients (n=27) were at advanced
stages of the disease at the moment of the diagnosis.
On the other hand, patients whose
images showed a benign aspect are still receiving annual follow-ups without
invasive methods, thus making the patient feel more at ease.
Only one of the patients
categorized as Lung-RADS 4 had a diagnosis of benignancy, of infectious origin;
he/she received specific treatment and didn’t show any alterations in
subsequent check-ups up to the end of this research.
Patients with nodules categorized
as Lung-RADS 4 had the possibility to undergo early treatÂment without any
subsequent check-ups or delays, and in some cases, they could use more specific
methods such as PET or lung biopsy to obtain an accurate anatomopathological
diagnosis before determining the approach to be used or before starting their
treatment such as chemotherapy or neoadjuvant therapy.
Pulmonary diseases, mostly lung
cancer, have become stronger in the last years due to some factors not
necessarily related to smoking: there are histological types of cancer that
affect not only smokers (for example, adenocarcinoma) but also non-smokers,
since environmental pollution, work and lifestyle are important factors with an
essential role in the development of the disease, apart from smoking. The
contribution of new technologies, for example the volumetric multi-slice
computed tomography of thin slices as well as the possibility to use low doses
of radiation force current physicians to put all their efforts into helping the
patient, with the aim of prolonging his/her survival and improving his/ her
quality of life, with the obligation to keep their knowledge up-to-date and use
such knowledge to provide information, education and the best patient care.
Thus, it is possible to have medical advances and include lung cancer in our
screening scheme using the suitable guidelines for the purpose of deÂtecting
this disease at early stages.
Approximately half of lung
cancers are presented as advanced disease as soon as they are diagnosed, with a
5-year average survival of 17%. Timely detection and optimum treatment of lung
cancers at their early stage are essential, since patients with localized
disease increase their 5-year survival to 55%. To do that, it is necessary to
use screening systems for cancer detection that are not yet established in
healthcare systems in Argentina, as indicated before. However, by applying the
criteria of the guidelines set for the categorization and follow-up of
pulmonary nodules incidentally discovered in conventional studies, it is
possible to contribute to good patient follow-ups with a higher probability of
detecting the malignant disease at early stages.
Conclusion
The application of the Lung-RADS
system to this series of patients has shown a good management of patients’
follow-up with surgical resections in some cases and an expectant approach in
others, providÂing certain security mostly avoiding the use of unnecessary
aggressive treatments.
 References
1. Yip R, Henschke C, Yankelevitz
D, Smith P. Thoracic Imaging: Alternative Definitions of Positive Test Result
at CT Lung Cancer Screening. Radiology 2014; 273(2): 591-6.
2. Sistema de datos e informes
pulmonares (Lung-Rads). Medical Criteria. http://medicalcriteria.com/web/es/lung-rads/.
Acceso en la web: 30/08/2019.
3. Kathlen L. Lung Cancer
Screening Update. J Thorac Imaging 2016; 31: 190-200.
4. Govert JA, Wahidi MM, Goudar
RK, Gould MK. Evidence for the Treatment of Patients With Pulmonary Nodules:
When Is It Lung Cancer? Journal Chest 2007; 132(3): 94-100.
5. Knipe H. Fleischner Society
pulmonary nodule recommendations: Guidelines 2017. Radiopaedia. https://radiopaedia.org/articles/fleischner-society-pulmonary-nodule-recommendations.
Acceso en la web: 12/06/2018.
6. Martin MD, Kanne JP, Broderick
LS, Constantine AG. The National Lung Screening Trial: Overview and Study
Design. Radiology 2011; 258(1): 243-53.
7. Wiener RS, Gould MK, Woloshin
S, Schwartz LM, Clark JA. What do you mean, a spot? a qualitative analysis of
patients’ reactions to discussions with their physicians about pulmonary
nodules. Chest 2013; 143(3): 672-7.
8. MacMahon H, Naidich N, Goo JM,
Lee KS, Leung A. Guidelines for Management of Incidental Pulmonary Nodules
Detected on CT Images: From the Fleischner Society 2017. Radiology 2017; 284(1): 230-1.
9. Kakinuma R, Muramatsu Y, Kusumoto
M, Tsuchida T, and cols. Solitary Pure Ground-Glass Nodules 5 mm or Smaller:
Frequency of Growth. Radiology 2015; 276 (3): 873-82.
10. Kazerooni EA, Meyer CA. Lung-RADS:
Pushing the Limits. RadioGraphics 2017; 37: 1975-93.
11. Di Muzio B, Morgan M. Lung-RADS. Radiopaedia https://radiopaedia.org/articles/lung-cancer-screening.
Acceso en la web: 04/03/2018.
12. Wu R. Lung tumor.
Dysplasia / carcinoma in situ. Bronchioloalveolar atypical adenomatous
hyperplasia (AAH). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lungtumorbronchAAH.html.
Acceso en la web: 02/10/2019.
13. Kitagawa H, Goto A, Niki T,
Hironaka M, Nakajima J, Fukayama M. Lung adenocarcinoma associated with
atypical adenomatous hyperplasia. A clinicopathological study with special
reference to smoking and cancer multiplicity. Pathology International 2003; 53(12):
826-7.
14. Chung K, Jacobs C, Scholten
E, Goo J , Prosch H, Col. Lung-RADS Category 4X: Does It Improve Prediction of
Malignancy in Subsolid Nodules? Radiology 2017; 284(1): 269-70.
15. McKee J, Regis S, McKee A,
Flacke S, Wald C. Performance of ACR Lung-RADS in a clinical CT lung screening
program. JACR 2016; 13(2): 25-9.
16. Sim YT, Goh YG, Dempsey MF, Has S, Poon FW. PET-CT evaluation of
solitary pulmonary nodules: correlation with maximum standardized uptake value
and pathology. Lung 2013; 191(6): 623-7.