Autor : Bosio MartĂn1, Young Pablo2, Finn Bárbara C2, Ernst Glenda1, Borsini Eduardo1, DĂ©cima Tamara1, Salvado Alejandro1
1 Pulmonology Service, Hospital Británico de Buenos Aires 2 Clinical Medicine Service, Hospital Británico de Buenos Aires
Correspondencia : Dr. Martin Bosio, Pulmonology Service, Hospital Británico, Perdriel 74, 1280 Buenos Aires, Argentina - e-Mail: drmarbosio@gmail.com
Abstract
Sarcoidosis is a systemic granulomatous inflammatory disease of unknown etiology and variable incidence. For the purpose of describing the clinical presentation of a group of patients diagnosed with sarcoidosis in a community hospital, we reviewed the medical records of patients whose diagnosis was consistent with sarcoidosis between 2007 and 2017. In this period, 24 patients were included and staged at presentation according to radiological data, showing that 75% were Stage I, 5% stage II, 10% stage III, and 10% stage IV. 60% of patients were treated.
This study describes the patients’ characteristics with the aim of helping to identify this entity and optimize early diagnosis and treatment.
Key words: Sarcoidosis; Prognosis; Epidemiology; Treatment; Pulmonary Function Tests.
Introduction
Sarcoidosis is a systemic granulomatous inflammatory disease of unknown etiology. It is characterized by the presence of non-caseating granulomas compromising the lungs, and mediastinal and hilar lymph nodes in more than 90% of the cases1-3. Although the first description was issued by Jonathan Hutchinson (1828-1913) in 1869, the origin of the word “sarcoidosis” (given its similarity with sarcomatous lesions; which means, in Greek “flesh like condition”) dates back to 1898, when Caesar Boeck (1845-1917), a Norwegian dermatologist, described skin nodules characterized by foci of epithelioid cells and giant cells, thus naming this benign skin condition. This term has been universally accepted since 19404,5.
This disease affects both men and women of all races and ages, though it predominates in young adults. Its frequency varies throughout the world, with a reported incidence between 5 and 40 cases per 100 000 inhabitants/year. More severe forms of this disease6-9 have been described in Scandinavian countries and within the African-American population.
There is low incidence in Latin America, maybe due to genetic differences and different environmental exposure to certain antigens. It can be confusing to make the diagnosis, due to the lack of data and high prevalence of other granulomatous diseases such as tuberculosis or deep mycoses. Some series of local cases have been published, however, we don’ have studies providing updated information10-14. Our purpose was to describe the clinical presentation of a cohort of patients diagnosed with sarcoidosis in a community hospital and to show the experience of our Center so that future research projects on this topic can be done.
Materials and Methods
We systematically reviewed the data of patients who were consecutively treated in our Center from December 2007 to March 2017. We used the WASOG (World Association of Sarcoidosis and Other Granulomatous Diseases) classification for the diagnosis of sarcoidosis14 meeting the required criteria, a clinical condition suggestive of sarcoidosis and a histopathologic analysis with presence of non-caseating granulomas, disregarding other causes of granulomatous lesions. The design of the study was retrospective and descriptive. This study was approved by the Institutional Review Board of the Hospital Británico de Buenos Aires in accordance with the Declaration of Helsinki.
Once the patients who fulfilled the eligibility criteria were identified, we searched their clinical records for information regarding clinical presentation and treatment and demographic data. The findings of chest X-ray, high resolution chest tomography (HRCT) and pulmonary function tests such as computed spirometry and carbon monoxide diffusing capacity (DLCO) were recorded.
The following information was also recorded: Scadding radiological staging, smoking, extrapulmonary involvement, presenting symptoms and biochemical analyses.
We used the Graph Pad Prism 7.04 program. Quantitative variables (continuous) were expressed in median ± interquartile range 25-75%; categorical variables (nominal) were expressed in percentages. Confidence intervals were calculated for 95% of the cases. For the association of categorical variables, we used the Fisher’s Exact Test; for quantitative variables, the Student’s T Test. A value of p < 0.05 was considered as significant.
Results
In this period, 24 patients were included. There weren’t any differences in the proportion of men and women, and the mean age of patients was 51.2 ± 17.3 years (30-78 range). Table 1.
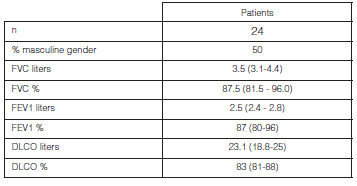
At the moment of the diagnosis, 80% of patients showed normal spirometry, 10% had moderate obstructive pattern and the remaining 10% showed restrictive pattern, also moderate. 70% of patients underwent a carbon monoxide diffusion test (DLCO), with normal results in 80% of the cases (Table 1).
The main presenting symptoms were dyspnea and cough in 30% of the cases, followed by asthenia in 20%, abdominal pain and hemoptisis in 5%. The Löfgren Syndrome was observed in 5% of the patients. Some patients showed more than one symptom, whereas 55% of them were asymptomatic. Extrapulmonary involvement was observed in 45% of patients, skin involvement in 25%, in most cases with erythema nodosum, mild pericardial effusion in 10% and pleural effusion in 5%. 10% of patients showed mild anemia, 25% had a moderately elevated erythrocyte sedimentation rate and 15% presented a high level of angiotensin-converting enzyme (ACE).
60% of patients had never smoked before, 25% were ex-smokers and 15% continued smoking.
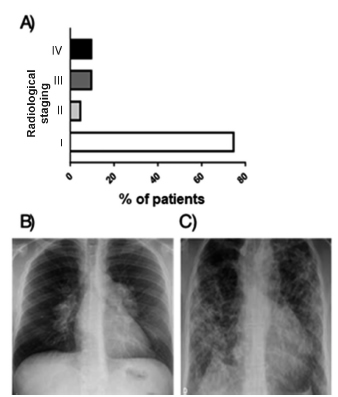
At presentation, 75% of patients were stage I (hilar and mediastinal adenopathies without pulmonary infiltrates), 5% were stage II (hilar adenopathies and pulmonary infiltrates mostly in upper areas), 10% stage III (pulmonary infiltrates mostly in the upper lobes without hilar lymph nodes) and 10% stage IV (pulmonary fibrosis with volume loss) (Figure 1).
The following screening tools were used: mediastinoscopy, 40% of the cases; videothoracoscopy, 20%; fibrobronchoscopy with transbronchial biopsy, 15%; skin biopsy, 10%; endobronchial ultrasound (EBUS), 5%, and liver biopsy, 5%, showing non-caseating granulomas in the biopsy sample. The clinical diagnosis of the remaining 5% was attributed to the Löfgren syndrome18.
60% of patients received corticosteroid treatment with meprednisone, the most frequent dose being 0.5 mg/kg. Such patients continue with outpatient management, with a good evolution. 28.5% showed disease relapse, all within one year after suspending corticosteroids. In every case we repeated the same treatment regime and symptoms improved with no need to modify the treatment.
Discussion
Despite the absence of a definite etiology, we maintain that the pathogenesis of sarcoidosis implies exposure to one or several environmental or non-environmental agents in genetically susceptible individuals6, 13. This combination causes the activation of the immune system components and the formation of non-necrotizing granulomas, the characteristic lesions of this disease. Depending on unknown genetic alterations or defects in the immune system, the granulomatous reaction may be solved or persist as chronic inflammation, finally leading to fibrosis. The different combinations of exposure + defects in the host determine the existence of the multiple phenotypes observed in sarcoidosis14. Newman et al conducted a case control etiologic study of sarcoidosis (ACCESS) to identify the existence of an occupational and environmental association15. They concluded that severe exposure in certain cases was associated with the risk of developing sarcoidosis, including insecticides, agricultural jobs and bioaerosols. Also, they concluded that smoking had a protective effect. Furthermore, some authors have suggested that association with Mycobacterium tuberculosis might be one of the potentially responsible infectious agents, since there are studies that detected the mycobacterial catalase-peroxidase protein (mKatG) in tissue samples of patients with sarcoidosis15-19. This protein has the same physical and chemical properties as Kveim Siltzbach, which has been used to induce skin granulomas in patients with sarcoidosis. The antigens of mycobacteria are released during the death of the organism20.
Sarcoidosis has better evolution than other interstitial pulmonary diseases. Its high spontaneous remission rate with little consequences and the chronic course of the disease occur in approximately one third of the patients. There is a reported mortality rate between 1 and 6%21-23.
In accordance with the International Consensus, diagnosis relies on three criteria: compatible clinical and radiological presentation, pathologic evidence of non-caseating granulomas with or without minimal central necrosis and exclusion of other diseases with similar findings7, 14.
Radiological staging proposed by K. Wurn in 1958 and then adopted by Scadding JG25 in 1961 derive from chest X-ray (CXR) and not from the high-resolution computed tomography (HRCT). Despite the existing discrepancy found in many patients between the findings of the CXR and the HRCT, pulmonary alterations haven’t been well studied in order to obtain the prognostic assessment of the disease with HRCT, thus, staging is made mainly in accordance with the findings of the X-ray film7, 25.
The diagnosis of sarcoidosis can only be made without a biopsy in patients with a specific clinical and radiological presentation: presence of hilar adenopathies in chest radiography of an asymptomatic patient; Lofgrens Syndrome: bilateral adenopathies in the CXR, erythema nodosum and arthritis; Heerfordt Syndrome: parotid gland enlargement and facial nerve palsy, anterior uveitis and fever; panda sign (parotid and lacrimal glands uptake) and lambda sign (right paratracheal and bilateral hilar lymph nodes uptake) in the scintigraphy with Galium-67.
It is important to point out that the evidence of granulomas in the pathologic anatomy only, without a compatible clinical context, is not enough for the diagnosis of sarcoidosis, since there are several other entities characterized by this type of inflammation, infection, hypersensitivity pneumonitis, chronic aspiration, berylliosis, lymphoproliferative disorders, pneumoconiosis, etc.
Various studies have suggested differences in the forms of presentation of sarcoidosis around the world. Pulmonary involvement occurs at some point in every patient with sarcoidosis; subclinical involvement is present, even when extrathoracic manifestations predominate. Non-productive cough, dyspnea and discomfort are present in 30% to 50% of patients; there is a substantial delay between the beginning of the symptoms and the diagnosis, approximately half of the patients are properly diagnosed more than 3 months after symptoms started26. In a cohort study in the United States including 448 patients with incidental sarcoidosis between 1976 and 2013, intrathoracic involvement was reported in 97% of patients; 87% showed intrathoracic adenomegalies and 50% had parenchymal involvement, even though 43% were asymptomatic27. There were also clinical, age-related differences at presentation: patients younger than 45 years with lymph node extrathoracic involvement showed greater involvement of salivary and parotid glands and liver, whereas cardiac, muscular, ocular and renal involvement was more frequent in patients older than 45 years28. In our series, most patients were asymptomatic,
thus being an incidental finding in patients being studied for non-specific symptoms who went to the Hospital emergency department. This could suggest the overuse of HRCT in our area, and that could explain the higher percentage of patients diagnosed at stage I and asymptomatic.
The rest of the most frequent clinical manifestations are erythema nodosum and few respiratory symptoms, such as non-productive irritating cough, thoracic pain and dyspnea, with clinical-radiological dissociation (greater radiological than clinical involvement)29. The skin and the eyes are the most common organs with extrapulmonary involvement; the heart and nervous system are the most severe; it is essential to monitor extrapulmonary involvement since early diagnosis may prevent the irreversible infection30.
The typical physiological abnormality is the restrictive ventilatory defect with reduction in carbon monoxide diffusion capacity (DLCO). An isolated mild reduction in DLCO is also frequent, however, obstruction to the airflow has been more prevalent in some cohort studies31.
Given that parenquimatic involvement is highly prevalent in sarcoidosis, the fibrobronchoscopy (FBC) has high diagnostic performance. Airway samples, endobronchial biopsies, pulmonary parenchyma, transbronchial biopsies and cryobiopsy or EBUS (endobronchial ultrasound) with intrathoracic lymph node sample may be included. The most common differential diagnoses (Scadding stage I) are the infectious ones such as tuberculosis, fungal and others such as lymphoma. When there are visible abnormalities in the FBC, the possibility to obtain a diagnosis is high, of more than 70%, but decreases to 30% if the mucosa is normal32.
Individual studies and a meta-analysis have shown that EBUS has more than 80% diagnostic accuracy. It has high sensitivity to detect granulomas, but low specificity for diagnosing sarcoidosis, since they may be confused with tuberculous lymphadenopathy and may be adjacent to lung cancer33-36.
The cryobiopsy and EBUS of 36 non-selected patients with suspicion of sarcoidosis showed 67% sensitivity for every separate procedure, but presented 100% sensitivity when the procedures were combined37.
In our analysis we reported skin conditions in 25% of the cases. Generally, skin conditions are early findings, mostly asymptomatic, though sometimes presented with pruritus. Most lesions can be treated with topical agents. The 2 most important lesions are erythema nodosum and lupus pernio. Since ocular involvement occurs through inflammation and is generally asymptomatic, annual eye exams are required to avoid permanent alterations. The most frequent presentations are anterior uveitis and keratoconjunctivitis, which may be treated with topical agents; severe anterior and posterior uveitis require systemic treatment38.
Even though cardiac sarcoidosis is observed in 25 to 70% of autopsies39, clinical cardiac involvement occurs in 5% of the patients of our series.
Neurological involvement occurs in less than 10% of patients, with the cranial pairs being the most frequent cases of involvement. The VII cranial nerve is the most frequently affected40.
The treatment is not indicated in asymptomatic stage I and II patients, since there is 60% to 90% spontaneous resolution after 2 years in stage I, and it is the form of presentation in 25% to 65% of the cases; remission in stage II is 40% to 70%, and the rest remains stable or may progress over time and findings are present at the initial diagnosis in 20% to 40% of the cases; in stage III, 10% to 20% of patients show spontaneous resolution and it is the form of presentation in 10% to 15% of the cases; finally, in stage IV there is no spontaneous resolution and it is the form of presentation in 5% of the cases7.
Corticosteroids are the main treatment but they do not cure the disease; immunomodulators and cytotoxic agents are generally used when corticosteroids fail or are not well-tolerated by the patient. There are no validated protocols or algorithms for oral treatment with corticosteroids. It should be considered in patients with significant or worsened symptoms, mainly dyspnea, alteration or impairment of the lung function (more than 10% of vital capacity or 15% or more DLCO reduction), and greater radiographic progression. Even in these cases the treatment is based on expert recommendations rather than the results of controlled randomized studies. A meta-analysis identified 13 studies with 1066 patients treated with systemic corticosteroids during 6 to 24 months. The authors found improvement
in symptoms and lung function in stage II and III41 patients. Therapeutic decisions should be based on quality of life and should be made taking into account every individual case.
Most stage I and II patients were diagnosed at the moment of the diagnosis, but half of them were treated with high doses of corticosteroids, in some cases indicated by physicians of other specialties without any previous interdisciplinary discussion. Knowing these characteristics and the adequate therapeutic management could contribute to the improvement of the clinical management of this disease.
Conflicts of interest: None.
1. Bargagli E, Prasse A. Sarcoidosis: a review for the internist (Review). Intern Emerg Med 2018; 13: 325-31.
2. Carmona EM, Kalra S, Ryu JH. Pulmonary Sarcoidosis: Diagnosis and Treatment (Review). Mayo Clin Proc 2016; 91: 946-54.
3. Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Müller-Quernheim J. Sarcoidosis (Review). Lancet 2014; 383: 1155-67.
4. Young P, Finn BC, Pellegrini D, Bruetman JE. Hutchinson (1828-1913), su historia, su tríada y otras tríadas de la medicina. Rev Med Chil 2010; 138: 383-7.
5. Fernández Fabrellas E. Epidemiología de la sarcoidosis (Review). Arch Bronconeumol 2007; 43: 92-100.
6. Newman LS, Rose CS, Maier LA. Sarcoidosis (Review). N Engl J Med 1997; 336: 1224-34.
7. Hunninghake G, Costabel U, Ando M, Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (WASOG) adopted by the ATS Board Directors and by the ERS Executive Committee. Am J Respir Crit Care Med 1999; 160: 736-55.
8. Rybicki BA, Major M, Popovich J, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol 1997; 145: 234-41.
9. Baughman RP, Teirstein AS, Judson MA, et al. ACCESS Research Group. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 2001; 164: 1885-9.
10. Bethlem NM. Epidemiology of sarcoidosis in Brazil. Sarcoidosis 1985; 2: 162.
11. Corrêa da Silva LC, Hertz FT, Cruz DB, et al. Sarcoidose no sul do Brasil: Estudo de 92 pacientes. J Bras Pneumo 2005; 31: 398-406.
12. Purriel P, Navarrete E. Epidemiology of sarcoidosis in Uruguay and other countries of Latin America. Am Rev Respir Dis 1961; 84: 155-61.
13. Song Z, Marzilli L, Greenlee BM, et al. Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med 2005; 201: 755-67.
14. Judson MA1, Costabel U, Drent M, et al. The WASOG Sarcoidosis Organ Assessment Instrument, Sarcoidosis Vasc Diff Lung Dis 2014 ; 31: 19-27.
15. Newman LS, Rose CS, Bresnitz EA A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med 2004; 170: 1324-30.
16. Semeniuk G, Bercovich C, Bernasconi D, Quesada Elias A, Schiavi E. Sarcoidosis. Revisión de 12 casos. Medicina (B Aires) 1983; 43: 257-62.
17. González EL, Vigliano C, Cáneva J. Sarcoidosis. Presentación clínica y pronóstico. Medicina (B Aires) 2010; 70: 499-502.
18. Cambursano H, Cazaux A, Langer M, et al. Sarcoidosis Broncopulmonar. Revisión breve y descripción de 8 casos. Rev Fac Cien Med Univ Nac Cordoba 2006; 63: 24-35.
19. Rodríguez Castells H, Carlos Rey J, Gustavo Dimier H, Valli EF. Encuesta de Sarcoidosis en la República Argentina-1969. Torax 1970; 19: 236-7.
20. Moller D, Potential etiologic agents in sarcoidosis. Dr Proc Am thorac Soc 2007; 4: 465-468.
21. Mañá J, Gómez-Vaquero C, Montero A, et al. Löfgren’s syndrome revisited: a study of 186 patients. Am J Med 1999; 107: 240-5.
22. Neville E, Walker A, James D. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med 1983; 52: 525-33.
23. Hunninghake G, Gilbert S, Pueringer R, et al. Outcome of the treatment for sarcoidosis. Am J Respir Crit Care Med 1994; 149: 893-8.
24. Scadding JG , Prognosisi of intrathoracic sarcoidosis in England. Br Med 1961: 2: 1165-72.
25. Lynch JP. Computed tomographic scanning in sarcoidosis. Semin Respir Crit Care Med 2003;24:393-418.
26. Judson MA, Thompson BW, Rabin D. The diagnostic pathway to sarcoidosis. Chest 2003; 123: 406-12.
27. Epidemiology of Sarcoidosis 1946–2013: A Population Based Study Ungprasert P, Carmona EM, Utz JP et al. Mayo Clin Proc. 2016; 91(2): 183-188.
28. Chevalet P, Clément R, Rodat O, Sarcoidosis diagnosed in elderly subjects: retrospective study of 30 cases Chest. 2004; 126: 1423-30.
29. Myers JL, Tazelaar HD. Challenges in pulmonary fibrosis: Problematic granulomatous lung disease. Thorax 2008; 63: 78-84.116 117
30. Rizzato G, Palmieri G, Agrati AM, Zanussi C. The organ-specific extrapulmonary presentation of sarcoidosis: a frequent occurrence but a challenge to an early diagnosis. A 3-year-long prospective observational study. Sarcoidosis Vasc Diffuse Lung Dis 2004; 21: 119-26.
31. Bussinguer M, Danielian A, Sharma OP. Cardiac sarcoidosis: diagnosis and management. Curr Treat Options Cardiovasc Med 2012; 14: 652-64.
32. Nunes H, Humbert M, Capron F, Pulmonary hypertension associated with sarcoidosis: mechanisms, haemodynamics and prognosis. Thorax 2006; 61: 68-74.
33. Shorr AF, Torrington KG, Hnatiuk OW , Endobronchial biopsy for sarcoidosis: a prospective study Chest 2001; 120: 109-14
34. Agarwal R, Srinivasan A, Aggarwal AN, Efficacy and safety of convex probe EBUS-TBNA in sarcoidosis: a systematic review and meta-analysis. Respir med 2012;106: 883-92.
35. Trisolini R, Lazzari Agli L, Tinelli C, , Endobronchial ultrasound-guided transbronchial needle aspiration for diagnosis of sarcoidosis in clinically unselected study populations. Respirology 2015; 20: 226-34,
36. Navani N, Molyneaux PL, Breen RA Utility of endobronchial ultrasound-guided transbronchial needle aspiration in patients with tuberculous intrathoracic lymphadenopathy: a multicentre study. Thorax 2011;66: 889-93
37. Aragaki-Nakahodo AA, Baughman RP, Shipley RT, The complimentary role of transbronchial lung cryobiopsy and endobronchial ultrasound fine needle aspiration in the diagnosis of sarcoidosis. Respir Med 2017; 131: 65-69.
38. Al-Kofahi K, Korsten P, Ascoli C et al. Management of extrapulmonary sarcoidosis: challenges and solutions. Ther Clin Risk Manag. 2016; 12: 1623-163.
39. Bussinguer M, Danielian A, Sharma OP. Cardiac sarcoidosis: diagnosis and management. Curr Treat Options Cardiovasc Med 2012; 14: 652-64.
40. Stern BJ. Neurological complications of sarcoidosis. Curr Opin Neurol 2004; 17: 311-6.
41. Paramothayan NS, Lasserson TJ, Jones PW. Corticosteroids for pulmonary sarcoidosis. Cochrane Database Syst Rev 2005; (2): CD001114.