Autor : De Vito, Eduardo L1,2,3, Tripodoro Vilma A.1
1Medical Research Institute Alfredo Lanari, Faculty of Medicine, University of Buenos Aires, Buenos Aires, Argentina. 2Centro del Parque, Respiratory Care Department, Buenos Aires, Argentina. 3Investigador Vinculado, NavarraBiomed, Centro de InvestigaciÃģn BiomÃĐdica, UPNA, EspaÃąa.
https://doi.org/10.56538/ramr.FFFU6290
Correspondencia : Eduardo Luis De Vito. E-mail: eldevito@gmail.com
ABSTRACT
The concept of breathlessness
emphasizes the multidimensional subjective nature of dyspnea with physical,
psychological, social, spiritual, and existential components. âTotal dyspneaâ
advocates a comprehensive, patient-oriented approach beyond the disease. The
term ârefractory dyspneaâ should be avoided; it implies a certain therapeutic
nihilism. For this reason, the term âchronic dyspnea syndromeâ was coined to
recognize treatment possibilities and raise awareness among patients,
physicians, healthcare teams, and researchers. People with advanced respiratory
disease and severe chronic dyspnea (and people who are close to them) have a
poor quality of life. The Breathing-Thinking- Functioning clinical-conceptual
model includes the three predominant cognitive and behavioral reactions that
worsen and maintain the symptom by causing vicious circles. Various instruments
are available for the comprehensive assessment of dyspnea that take into
account the sensory-perceptual experience, affective distress, and the impact
or burden of the symptoms. Breathlessness during the last weeks or days of life
can be called âterminal dyspnea.â It is a common symptom and one of the most
distressing in the latest phase of life of patients with cancer. Under these
circumstances, self-report may underestimate respiratory distress. The
Respiratory Distress Observation Scale is the first and only dyspnea assessment
instrument designed to evaluate its presence and intensity in patients who are
unable to communicate.
Key words: Breathlessness, Total dyspnea, Refractory dyspnea, Chronic
dyspnea syndrome, Terminal dyspnea
RESUMEN
El concepto de âdificultad para respirarâ enfatiza la
naturaleza subjetiva multidimensional de la disnea con componentes
físicos, psicológicos, sociales, espirituales y existenciaÂles.
La âdisnea totalâ aboga por un enfoque integral y centrado en el paciente
más allá de la enfermedad. La noción de âdisnea
refractariaâ debería ser evitada; implica cierto nihilismo
terapéutico. Por tal motivo, se acuñó el concepto de
âsíndrome de disnea crónicaâ para reconocer las posibilidades de
tratamiento y concientizar a los pacientes, los médicos, el equipo de
salud y los investigadores. Las personas que viven con enferÂmedades
respiratorias avanzadas y disnea crónica grave (y sus allegados) tienen
una mala calidad de vida. El modelo clínico-conceptual
ârespirando-pensando-funcionandoâ incluye las tres reacciones cognitivas y
conductuales predominantes que, al provocar círculos viciosos, empeoran
y mantienen el síntoma. Para la evaluación exhaustiva de la
disnea se dispone de diversos instrumentos que consideran la experiencia
sensorial-perceptiva, la angustia afectiva (distress) y el impacto o
carga de los síntomas. La dificultad para respirar durante las
últimas semanas o días de vida puede denominarse âdisnea
terminalâ. Es un síntoma frecuente y uno de los más angustiantes
en la última fase de la vida de pacientes con cáncer. En estas
circunstancias el autorreporte puede subestimar la dificultad respiratoria. La
Escala de Observación de Dificultad Respiratoria es el primer y
único instrumento de evaluación de la disnea destinado a evaluar
su presencia e intensidad en pacientes que no pueden comunicarse.
Palabras clave: Dificultad para respirar, Disnea total, Disnea refractaria, Síndrome
de disnea crónica, Disnea terminal
Received: 01/08/2023
Accepted: 05/02/2024
MULTIDIMENSIONAL APPROACH TO DYSPNEA
The concept of âbreathlessnessâ
is widely used in the literature related to palliative care, instead of the
biomedical term âdyspneaâ, to emphasize the daily experience from the patientâs
perspective.1 The multidimensional subjective nature of dysÂpnea goes beyond
merely the physical condition and highlights the importance of the psychologiÂcal,
social, spiritual, and existential components. Similar to the concept of âtotal
painâ described by Dame Cicely Saunders in the early 1960s, the concept of
âtotal dyspneaâ has been suggested, adÂvocating for a comprehensive and
patient-centered approach beyond the disease itself.2
If dyspnea persists despite
treatment of the underlying disease, it is sometimes referred to as ârefractory
dyspnea.â Since this term implies therapeutic nihilism, Johnson et al suggested
callÂing it âchronic dyspnea syndromeâ to acknowledge treatment possibilities
and raise awareness among patients, physicians, healthcare teams, and reÂsearchers.2 Chronic
dyspnea syndrome is described as the sensation of breathlessness that
persists despite receiving optimal treatment of the underlyÂing physiopathology
and causes disability.3-5 Like total
pain, total dyspnea encompasses multiple aspects and incorporates previously
neglected constructs, such as the suffering related to the meaning patients
assign to their symptoms. Total pain includes four domains: physical,
psychologiÂcal, interpersonal, and existential, and these are assessed from the
patientâs perspective. Dyspnea is subjected to a similar analysis.6
For more than a decade, the
respiratory sensaÂtion (neural activation resulting from the activaÂtion of
peripheral receptors) and perception (conÂscious and individual reaction to the
sensation) have been clearly differentiated.7
Consequently, the sensation of dyspnea
doesnât need to be related to identifiable physiological factors. Patientsâ exÂperiences
with dyspnea vary widely depending on factors such as ethnicity, experiences
with other diseases, and emotional state. Additionally, various psychological
and cultural factors can influence the reaction to a sensation. A stoic
individual may not perceive (or deny) respiratory discomfort. The context in
which the sensation occurs can also modify the perception of the sensory
experience.8-10
Anxiety, a particularly common
psychological factor that correlates with dyspnea, can exacerÂbate the symptom,
leading to a progressive spiral of increased dyspnea and higher distress.11,12 EpiÂsodes of
dyspnea can be predictable with known triggering factors, such as exertion,
emotions, comorbidities, or the external environment, or they can appear to be
unpredictable. Linde et al stated that many seemingly inexplicable episodes
were known to have been driven by fear or panic when evaluated in depth.13
IMPACT OF DYSPNEA
Dyspnea often triggers panic,
fear, anxiety, deÂpression, hopelessness, a sense of loss of control, and a
feeling of imminent death.14,15 It also affects daily and social functions,
leading to dependency and loss of roles. This symptom is one of the six
parameters used in the Palliative Prognostic Score, which predicts the 30-day
survival of patients in palliative care.16
A qualitative study illustrated
the meaning of the dyspnea experience in patients with cancer, COPD (chronic
obstructive pulmonary disease), heart failure, and amyotrophic lateral
sclerosis (ALS):17
â For individuals with cancer,
dyspnea serves not only as a sign of the presence of cancer but also as a
reminder of mortality, even in the face of treatment optimism.
â In people with COPD, dyspnea is
perceived as self-inflicted due to lifelong smoking.
â For those with heart failure,
dyspnea is associÂated with functional limitations and contributes to the
negative effects of other symptoms.
â In individuals with ALS, dyspnea
is related to mechanisms that are essential for living.
DYSPNEA AND QUALITY OF LIFE
People with advanced respiratory
diseases and severe chronic dyspnea (and the people close to them) have a poor
quality of life.1,17 Chronic dyspnea is an incapacitating symptom,
and acute episodes are terrifying to experience and observe. Many millions of
people around the world live with advanced respiratory diseases, such as COPD
and interstitial lung disease (ILD).18
These individuals arenât receiving the
palliative care they need to have the best possible quality of life.
These prevalent diseases, as well
as other less common ones, can progress rapidly or have a more chronic course,
and younger patients may be awaitÂing a lung transplant. However, in all cases,
as the disease progresses, the patient becomes very symptomatic and is unlikely
to improve despite maximum treatment of the underlying disease.
Although there is no comparable
infrastructure for providing palliative care in non-oncological respiratory medicine,
it is recognized that the deficit in symptom control and psychosocial supÂport
leads to a poorer quality of life for patients not only with advanced
respiratory diseases but also in the general population, and it is likely to
have a negative effect on medical outcomes.19
ACUTE AND CHRONIC DYSPNEA AND PERSON-CENTERED CARE
Generally, more attention is
given to the treatÂment of acute dyspnea than to chronic dyspnea.
Therefore, it is the responsibility of every physician to provide the best person-centered
care possible, ensuring the use of all available resources and knowledge.19
The term person-centered care,
recognized as a hallmark of excellence in care, also encompasses the choice of
specific treatments targeted at the disease, and provides a foundation for
ensuring that patients receive individualized treatment, and that their
symptoms and other concerns are identified. Healthcare professionals must play
an active role in promoting quality of life as part of excellent medical care.
DYSPNEA IN TERMINAL RESPIRATORY DISEASE
The cardinal symptoms of advanced
respiratory disease are persistent dyspnea, fatigue, and cough, sometimes
referred to as the respiratory triad.20, 21 All these symptoms
can be invisible at rest, so they need to be actively elicited to detect them.
Scientific evidence for symptomatic treatments has improved in recent years;
not using them in specialized respiratory care services should be considered
inexcusable.
Other factors that greatly affect
the outcomes and quality of life are outside the sphere of clinical influence,
for example: financial anxiety, housing loss, lack of food, difficulty
obtaining social asÂsistance, concern about the continuity of health benefits,
social isolation, caregiver burnout, and breakdowns in family relationships.
Being outside the sphere of clinical influence does not mean they should be
ignored.
THE EXISTENTIAL MEANING OF DYSPNEA
The spiritual repercussions of
dyspnea can be strong. Just like pain, patients may attribute a variety of
meanings to dyspnea, ranging from Godâs punishment to a divine gift.
Additionally, the patientâs religious or metaphysical beliefs can influence the
extent of their suffering.22-24 The secondary physiological and behavioral responses to
dyspnea should be considered in the evaluaÂtion process. This concept has been
subjected to experimental testing, supporting the hypothesis that the multiple
dimensions or components can be measured as different entities. Some studies
have demonstrated the existence of a separable âaffective dimensionâ
(i.e., distress and emotional impact).25
SUBJECTIVE INDIVIDUAL EXPERIENCE
Dyspnea affects people in
different ways and across different dimensions, which is well demonstrated by
the Breathing, Thinking, Functioning clinical model.21
This model conceptualizes the three preÂdominant cognitive and
behavioral reactions to dyspnea that, by creating vicious cycles, worsen and
sustain the symptom (Figure 1).
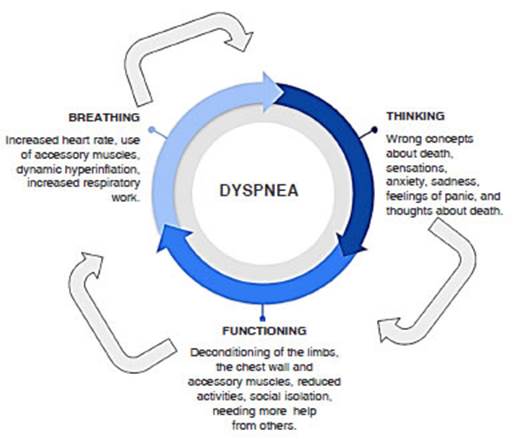
Breathing domain: shortness of breath asÂsociated with dysfunctional breathing patterns,
increased respiratory rate, the need to use accesÂsory muscles, and dynamic
hyperinflation, leading to inefficient breathing and increased respiratory
work.
Thinking
domain: misconceptions about the nature of dyspnea, such as
its cause, and previous experiences and memories that profoundly impact the
current experience. This negative
cognition and memories can cause anxiety, sadness, panic, and thoughts about
death.
Functioning domain: individuals suffering from dyspnea often reduce their physical activity
to avoid the sensation of breathlessness, leading to social isolation,
increased dependence on others, and general deconditioning.
FAMILY IMPACT OF DYSPNEA
The dyspnea experienced by
patients also afÂfects informal caregivers (family and friends). However, their
burden and anxieties are often overlooked and the existence of those feelings
is normalized.28
Caregivers of dyspnea patients
perform many invisible caregiving tasks, such as hygiene, dressÂing, symptom
control, and administering medicaÂtion and oxygen, in addition to all the
household chores.29 They also provide emotional support not only during the day
but also through diffiÂcult nights. They remain awake monitoring the patientâs
breathing, and checking if they are still alive. Thus, it is not surprising for
caregivers to report poor quality sleep, similar to people working shifts and
mothers of young children.
Therefore, when treating patients
with dyspnea, it is also necessary to focus on caregivers and their needs,
providing them with education, support, and resources, just like for the
patients. Caregivers should be encouraged to also take care of their own
health, both physical and emotional.
EVALUATION AND IDENTIFICATION OF THE CAUSES OF DYSPNEA
The assessment of dyspnea must
include the paÂtientâs subjective experience; therefore, the self-report of the
symptom is essential. This should include the sensory component with its
intensity and severity, the emotional burden reflected in the discomfort caused
by dyspnea, and the impact on the patientâs daily life.
Currently, there is no
universally accepted measure of health outcomes in clinical or research
settings, which poses a clear barrier to routine clinical evaluation and
follow-up of dyspnea.
The evaluation of a patient
should also include a physical examination with vital signs and a cardioÂpulmonary
exam. Although additional diagnostic tests may be important to identify any
treatable cause of dyspnea, it should be noted that more obÂjective
evaluations, such as respiratory rate, blood gas analysis, or lung function
tests do not meaÂsure dyspnea and only moderately correlate with the patientâs
subjective experience. For optimal management of dyspnea, treating the
underlying disease and addressing reversible symptoms are the first step.
INSTRUMENTS FOR MEASURING DYSPNEA
For a comprehensive evaluation of
its multidimenÂsionality, an instrument must reflect each patientâs subjective
sensory experience, provide an objective measurement of dyspnea, and facilitate
the effecÂtive communication with the healthcare team.31
There are three domains of dyspnea
measurement proposed by the American Thoracic Society in 2012 and 2013.11,12
1. Sensory-perceptual experience: this inÂcludes the ratings of symptom intensity, frequency, duration,
and sensory quality. The intensity of dysÂpnea can be assessed using a Visual
Analog Scale (VAS), the Borg Scale32,
Likert-type ratings, or the Numeric Analog Scale (NAS). Dyspnea is also includÂed
in validated Spanish multidimensional assessment tools, such as the Memorial
Symptom Assessment Scale and the Edmonton Symptom Assessment Scale (ESAS-r
validated in Spanish).33,34. For sensory qualÂity, Simon et al35
reported 15 descriptors of dyspnea used by patients in eight groups (rapid,
exhalation, shallow, effort, choking, hunger, tight, and heavy). The authors
suggested possible associations of the descriptors with specific conditions
causing dyspnea, but a subsequent study by Wilcock et
al36 could not
demonstrate the robustness of these descriptors in helping with the diagnosis
when applied to patients with cancer and cardiopulmonary diseases.37
2. Affective distress: this can refer to immediÂate distress or the distress that patients feel
when they understand the meaning or consequences of their symptom. Discomfort
can be rated as a single item, as in the case of dyspnea intensity. Scales with
multiple items, such as the one for dyspnea in cancer, assess emotional
responses, including anxiety.38,39
Two validated measures, Dyspnoea
12 and the Multidimensional Dyspnea Profile (MDP), are suitable for
use in both clinical and research settings and are brief enough for assessment
and follow-up.38-40
Dyspnoea 12 is a 12-item measure of dyspnea severity with 7 physical
items and 5 affective items not related to physical activity. Response options
are: none (0), mild (1), moderate (2), or severe (3). The time period refers to
âthese days.â40, 41
The MDP is also a
12-item scale that uses scores (0-10). Seven items refer to overall intensity,
unpleasantness, and other qualitative sensory descriptions of dyspnea
(increased respiratory muscle work, chest tightness, air hunger, breathÂing a
lot, requiring mental effort), and five rate the emotional responses to dyspnea
(depression, anxiety, frustration, anger, and fear).42,43
3. Impact or burden
of symptoms: this includes the
effect of dyspnea on behavior, funcÂtions, quality of life, or health status.
The Medical Research Council (MRC) scale provides a unidiÂmensional rating of
disability,44-46 while the
Chronic Respiratory Disease Questionnaire (CRQ) is a multidimensional scale
used for the assessment of functionality.47-49
The MRC scale
evaluates the consequences of dyspnea in relation to functional limitations. It
is particularly useful if dyspnea is the only symptom experienced by the
patient but is too insensitive to detect subtle changes after an intervention.8
Several quality of life scales have been validated for use in cancer or
specifically for lung cancer and chronic lung diseases, such as the CRQ47,48,50,51, EuroQol 5D, FACT-L52,
QLQ-C15-PAL53-55 and QLQ-LC13.56,57 Table 2 summarizes
the dyspnea assessment instruments and their main domains.
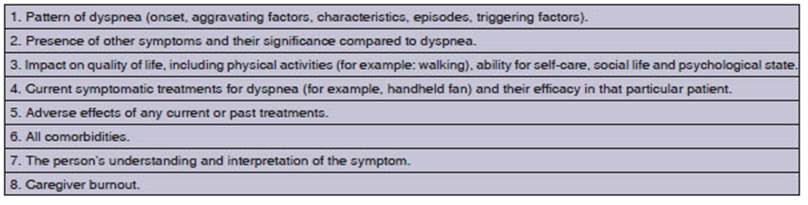
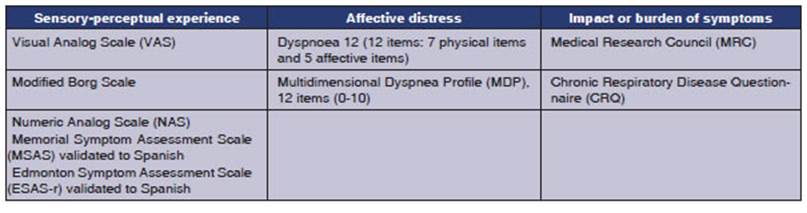
Currently, there are
more than 50 scales for measuring dyspnea, covering several dimensions and
different diseases.11 However, there is no unified measurement tool
for clinical use in the palliative care setting. It may be possible to comÂbine
the assessment of dyspnea intensity with its impact on the patientâs quality of
life.58 The NAS, the Modified Borg Scale, the CRQ dyspnea scale59,
the ALS Assessment Scale (based on the CRQ)60 and the Cancer Dyspnea
Scale seem to be the most suitable for the palliative care settings.61
Dyspnea can also be
measured in terms of exÂercise tolerance. Several instruments have been
validated in advanced disease, namely, the Shuttle Walking Test62,
the Reading Numbers Aloud Test63, and the Upper Limbs Test.64
Unfortunately, the fact that these tests are slow and expensive curÂrently
limits their applicability in the clinical setting.58
EVALUATION OF DYSPNEA CRISES
During crises, the
patient is likely to experience an increase in intensity, distress, and fear.12
Initially, dyspnea is often a component of several
symptoms, including depression and anxiety. Self-report is the most valid and
reliable method for assessing the patientâs experience, symptom progression,
and response to treatment.
The simplest
self-report is a dichotomous yes or no response to the question, âAre you
short of breath?â However, yes or no responses are unlikely to help with
palliative care, so the use of some rating of dyspnea intensity is expected. At
least a standardized measure is recommended, such as the 0-10 Numerical Rating
Scale, augmented by the evaluation of the patientâs subjective experience of
distress and discomfort alongside the rating of dyspnea intensity.
TERMINAL DYSPNEA
Dyspnea suffered by
patients during the last weeks or days of their life can be called âterminal
dyspnea.â65 Timely assessment and proactive management of
dyspnea in these patients are of vital importance. This symptom has unique
cliniÂcal characteristics: it tends to worsen rapidly over days or hours as
death approaches, even despite symptom control measures. The family is also
deeply affected, experiencing anxiety, uncertainty, helplessness, and
inability. An assessment of the family-caregiver, the need for information, the
desired level of participation in care, and home resources will support
caregivers and integrate them into the healthcare team.
International
guidelines have recently recogÂnized that dyspnea is common and one of the most
distressing symptoms in the final stages of life for cancer patients66
and that patients have difficulty self-reporting the symptom. Since self-report
is the gold standard for assessing dyspnea, there is a need to seek
alternatives for this group of patients.
In clinical practice,
healthcare teams conduct indirect assessments of symptom severity. HowÂever,
the accuracy of these subjective assessments may be poor. Studies conducted in
advanced cancer populations and in intensive care units show that indirect
assessments by physicians and nurses are significantly lower and poorly related
to patient self-report. Given the fact that symptom severity assessments
influence treatment decisions, a betÂter method for assessing dyspnea in
patients who cannot express themselves is necessary.
The Respiratory
Distress Observation Scale (RDOS) is an ordinal 8-item scale designed to
measure the presence and intensity of breathlessÂness in adults. This scale was
developed from a bio-behavioral framework by Campbell.67,68
It is the first and only dyspnea assessment tool developed so far to evaluate
its presence and intensity in paÂtients who are unable to communicate.68-70
There are two modifications of the RDOS available for intensive care patients:
the IC-RDOS (Intensive Care Respiratory Distress Observation Scale)68,69,71
and the MV-RDOS (Mechanical Ventilation RespiÂratory Distress Observation Scale).72
The RDOS demonstrates
strong inter-rater reliÂability, convergent validity, and divergent validity,
suggesting its reliability and validity as an assessÂment tool for palliative
care patients.
Most importantly, it
shows clinical utility by demonstrating good discriminative properties in
detecting patients with moderate and severe dyspnea. A new RDOS cutoff point of
≥ 3 has been proposed for therapeutic intervention, with a sensitivity of
68% and a specificity of 77% for distinguishing between moderate to severe
distress versus none (positive predictive value: 0.85 and negative predictive
value: 0.61).69,70 Table 3 shows the objective variables considered
by the RDOS and the corresponding scores.
Seven patients (63.6 %) completed
treatment, three patients (27 %) discontinued treatment, and one patient (9 %)
passed away during the treatment. In terms of follow-up, one patient showed
rifampicin resistance (patient with conÂcurrent lung involvement), and another
patient (9 %) experienced reversible hepatotoxicity due to pyrazinamide.
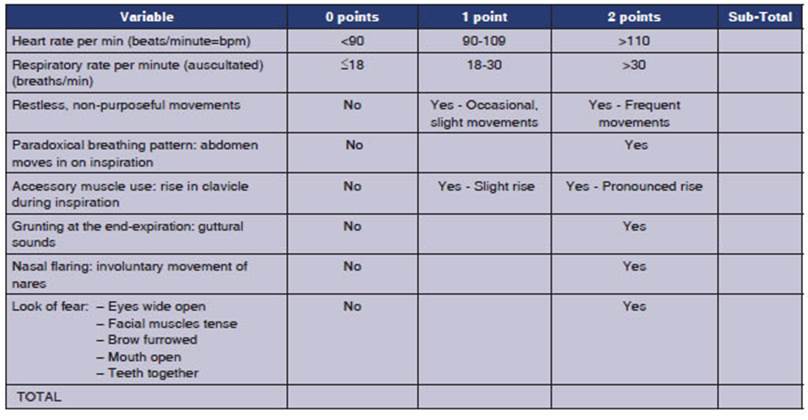
The evaluation of the
use of accessory muscles is based on the observation of the clavicle elevaÂtion
during inspiration. It does not refer to the palpation of accessory muscles
such as the scalene, intercostals, sternocleidomastoid, or the contracÂtion of
the abdominal muscles during expiration. Clavicle elevation somewhat reflects
the activation of the upper thoracic and neck muscles and is an easy-to-
identify sign.
The prevalence and
severity of dyspnea increase at the end stages of life. Many of the patients in
that situation have difficulty reporting their symptoms. Dyspnea is a symptom,
only the perÂson experiencing it can say that they are short of breath. Certain
operational aspects of the use of Table 3 should be emphasized:
â The RDOS is used in
adults and is not a subÂstitute for what the patient reports, as long as they
can express it.
â The scale should
not be used if the patient is paralyzed with a neuromuscular blocker and is not
valid in cases of bulbar ALS or quadriplegia.
â Respiratory and
heart rate can be measured during auscultation if necessary.
â Grunting can be
audible even in intubated paÂtients through auscultation.69
The RDOS is promising
and has clinical utilÂity as an observational dyspnea assessment tool. Further
studies in less communicative patients are needed to determine clinical utility
and the generalizability of the results.
CONCLUSIONS AND FUTURE APPROACH
It is not possible to
address a person with dyspnea without considering its multidimensional subjecÂtive
nature, with its physical, psychological, social, spiritual, and existential
components. The term âtotal dyspneaâ encompasses these components. The term
ârefractory dyspneaâ should be avoided and replaced by âchronic dyspnea
syndromeâ in order to recognize treatment possibilities and raise awareness
among patients, physicians, healthcare teams, and researchers.
â People living with advanced
respiratory diseases and severe chronic dyspnea (and the people close to them)
have a poor quality of life.
â The
Breathing-Thinking-Functioning clinical model includes the three predominant
cognitive and behavioral reactions against dyspnea that worsen and maintain the
symptom.
â There are various instruments
available for the comprehensive assessment of dyspnea as a multidimensional
symptom. These instruments propose considering the sensory-perceptual
experience, affective distress, and the impact or burden of the symptoms.
â Dyspnea suffered by patients
during the last weeks or days of their life can be called âtermiÂnal dyspnea.â
It is a common symptom and one of the most distressing in the latest phase of
life of patients with cancer.
â While the self-report of the
sensation of breathÂlessness is essential, some patients may not be able to
express it, and it may be underestimated. The Respiratory Distress Observation
Scale is the first and only dyspnea assessment instruÂment used to evaluate its
presence and intensity in patients who are unable to communicate.
A better understanding of the
brain processes underlying the perception of dyspnea will lead to new
therapeutic approaches aimed at improving the quality of life for a very large
group of patients.
REFERENCES
1. Booth S, Johnson MJ. Improving the quality of life of people with advanced respiratory
disease and severe breathlessness. Breathe (Sheff) 2019;15:198-215. https://doi.org/10.1183/20734735.0200-2019
2. Johnson MJ, Currow DC, Booth
S. Prevalence and assessÂment of breathlessness in the clinical setting. Expert
Rev Respir Med 2014;8:151-61.
https://doi.org/10.1586/17476348.2014.879530
3. Carel H, Macnaughton J, Dodd
J. Invisible sufferÂing: breathlessness in and beyond the clinic. Lancet Respir
Med 2015;3:278-9. https://doi.org/10.1016/S2213-2600(15)00115-0
4. Marjolein G, Higginson IJ.
Access to Services for PaÂtients with Chronic Obstructive Pulmonary Disease:
The Invisibility of Breathlessness. J Pain Symptom ManÂage 2008;36:451-60. https://doi.org/10.1016/j.jpainsymÂman.2007.11.008.
5. Morélot-Panzini C,
Adler D, Aguilaniu B, et al. DysÂpnoea working group of the
Société de Pneumologie de Langue Française. Breathlessness
despite optimal pathophysiological treatment: on the relevance of beÂing
chronic. Eur Respir J 2017;50:1701159.
https://doi.org/10.1183/13993003.01159-2017.
6. Langford RM. Pain:
nonmalignant disease for current opinion in supportive and palliative care.
Curr Opin SupÂport Palliat Care 2008;2:114-5.
https://doi.org/10.1097/SPC.0b013e32830139d5.
7. Guz A. Brain, breathing and
breathlessness. Respir Physiol 1997;109:197-04.
https://doi.org/10.1016/S0034- 5687(97)00050-9
8. Mahler DA, OâDonnell DE.
Dyspnea: Mechanisms, MeaÂsurement, and Management, Third Edition. CRC Press, 2014. https://doi.org/10.1201/b16363
9. Mahler, Donald (1991). Dyspnea. Medicine & SciÂence in Sports & Exercise
1991;23:1322.
https://doi.org/10.1249/00005768-199111000-00027.
10. Mahler DA. Understanding
mechanisms and documentÂing plausibility of palliative interventions for
dyspnea. Curr Opin Support Palliat Care 2011;5:71-6.
https://doi.org/10.1097/SPC.0b013e328345bc84
11. Parshall MB, Schwartzstein
RM, Adams L, et al. An offiÂcial American Thoracic Society statement: update on
the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care
Med 2012;185:435-52.
https://doi.org/10.1164/rccm.201111-2042ST
12. Mularski RA, Reinke LF,
Carrieri-Kohlman V, et al. An OfÂficial American Thoracic Society Workshop
Report: AssessÂment and Palliative Management of Dyspnea Crisis. Ann Am Thorac
Soc 2013;10:S98-S106.
https://doi.org/10.1513/AnnalsATS.201306-169ST
13. Linde P, Hanke G, Voltz R,
Simon ST. Unpredictable episodic breathlessness in patients with advanced
chronic obstructive pulmonary disease and lung cancer: a qualitaÂtive study.
Support Care Cancer 2018;26:1097-104.
https://doi.org/10.1007/s00520-017-3928-9.
14. Gysels M, Bausewein C,
Higginson IJ. Experiences of breathlessness: A systematic review of the
qualitative literature. Palliat Support Care 2007;5:281-302.
https://doi.org/10.1017/S1478951507000454
15. Simon ST, Higginson IJ,
Benalia H, et al. Episodes of breathÂlessness: Types and patterns â a
qualitative study exploring experiences of patients with advanced diseases. Palliat Med 2013;27:524-32.
https://doi.org/10.1177/0269216313480255
16. Pirovano M, Maltoni M, Nanni O, et al. A New Palliative Prognostic Score. J Pain Symptom Manage 1999;17:231-9.
https://doi.org/10.1016/S0885-3924(98)00145-6
17. Gysels M, Higginson IJ.
Access to services for patients with chronic obstructive pulmonary disease: the
invisibility of breathlessness. J Pain Symptom Manage 2008;36:451-60.
https://doi.org/10.1016/j.jpainsymman.2007.11.008
18. World Health Organization. Chronic respiratory diseases. https://www.who.int/health-topics/chronic-respiratory-diseases#tab=tab_1.
2023.
19. Currow DC, Dal Grande E, Ferreira D, et al. Chronic breathlessness associated with poorer physical and mental
health-related quality of life (SF-12) across all adult age groups. Thorax 2017;72:1151-3. https://doi.org/10.1136/thoraxjnl-2016-209908
20. Yorke J, Lloyd-Williams M,
Smith J, et al. Management of the respiratory distress symptom cluster in lung
cancer: a randomized controlled feasibility trial. Support Care Cancer 2015;23:3373-84. https://doi.org/10.1007/s00520-015-2810-x
21. Yorke J, Johnson MJ, Punnett G, et al. Respiratory distress symptom intervention
for non-pharmacological manageÂment of the lung cancer
breathlessnessâcoughâfatigue symptom cluster: randomised controlled trial. BMJ
SupÂport Palliat Care 2022;spcareâ2022.
https://doi.org/10.1136/spcare-2022-003924
22. Langford RM. Pain:
nonmalignant disease for current opinion in supportive and palliative care.
Curr Opin SupÂport Palliat Care 2008;2:114-5. https://doi.org/10.1097/SPC.0b013e32830139d5120
23. Booth S. Science Supporting
the Art of Medicine: ImÂproving the Management of Breathlessness. Palliat Med
2013;27:483-5.
https://doi.org/10.1177/0269216313488490.
24. Hutchinson, A. Patient and
carer experience of breathÂlessness. In C. Bausewein, D. C. Currow, & M. J.
JohnÂson (Eds.), Palliative Care in Respiratory Disease 2016 (102-110). European Respiratory Society. https://doi.org/10.1183/2312508X.10011615
25. Lansing RW, Gracely RH,
Banzett RB. The multiple dimensions of dyspnea: review and hypotheses. Respir
Physiol Neurobiol 2009;167:53-60.
https://doi.org/10.1016/j.resp.2008.07.012
26. Spathis A, Booth S, Moffat C,
et al. The Breathing, ThinkÂing, Functioning clinical model: a proposal to
facilitate evidence-based breathlessness management in chronic respiratory
disease. NPJ Prim Care Respir Med 2017;27:27.
https://doi.org/10.1038/s41533-017-0024-z.
27. Spathis A, Burkin J, Moffat
C, et al. Cutting through complexity: the Breathing,
Thinking, Functioning cliniÂcal model is an educational tool that facilitates
chronic breathlessness management. NPJ Prim Care Respir Med
2021;31:25.
https://doi.org/10.1038/s41533-021-00237-9.
28. Veloso VI, Tripodoro VA. Caregivers burden in palliaÂtive care patients: a problem to tackle.
Curr Opin SupÂport Palliat Care 2016;10:330-5.
https://doi.org/10.1097/SPC.0000000000000239.
29. Farquhar M. Supporting
informal carers. Palliative Care in Respiratory Disease.
Eur Respir Monogr 2016;73:51-69. https://doi.org/10.1183/2312508X.10011315
30. Bruera E, Higginson EJ, von
Gunten CF, Morita T. TextÂbook of Palliative Medicine and Supportive Care 2021,
CRC Press, https://doi.org/10.1201/9780429275524.
31. Chan K-S, Tse DMW, Sham MMK. Dyspnoea and other respiratory symptoms in palliative care. Oxford Textbook of Palliative Medicine 2015;421-34.
https://doi.org/10.1093/med/9780199656097.003.0082
32. Borg GAV. Psychophysical
bases of perceived exerÂtion. Med Sci Sports Exer 1982;14:377-81.
https://doi.org/10.1249/00005768-198205000-00012
33. Carvajal A, Hribernik N, Duarte E, et al. The Spanish Version of the Edmonton Symptom Assessment System- Revised
(ESAS-r): First Psychometric Analysis Involving Patients with Advanced Cancer.
J Pain Symptom ManÂage 2013;45:129-36.
https://doi.org/10.1016/j.jpainsymÂman.2012.01.014
34. Llamas-Ramos I, Llamas-Ramos
R, Buz J, et al. Construct Validity of the Spanish Versions of the Memorial
SympÂtom Assessment Scale Short Form and Condensed Form: Rasch Analysis of
Responses in Oncology Outpatients. J Pain Symptom Manage 2018;55:1480-91.
https://doi.org/10.1016/j.jpainsymman.2018.02.017
35. Simon PM, Schwartzstein RM,
Weiss JW, Fencl V, TeghtÂsoonian M, Weinberger SE. Distinguishable Types of
Dyspnea in Patients with Shortness of Breath. Am Rev Respir Dis 1990;142:1009-14. https://doi.org/10.1164/ajrccm/142.5.1009
36. Wilcock A, Crosby V, Hughes
A, Fielding K, Corcoran R, Tattersfield AE. Descriptors of
breathlessness in patients with cancer and other cardiorespiratory diseases.
J Pain Symptom Manage. 2002;23:182-9.
https://doi.org/10.1016/S0885-3924(01)00417-1
37. Chowienczyk S, Javadzadeh S,
Booth S, Farquhar M. AsÂsociation of Descriptors of Breathlessness With Diagnosis and Self-Reported Severity of Breathlessness
in Patients With Advanced Chronic Obstructive Pulmonary Disease or Cancer. J
Pain Symptom Manage 2016;52:259-64.
https://doi.org/10.1016/j.jpainsymman.2016.01.014
38. Tanaka K, Akechi T, Okuyama
T, Nishiwaki Y, Uchitomi Y. Development and validation of the Cancer Dyspnoea
Scale: a multidimensional, brief, self-rating scale. Br J Cancer 2000;82:800-5. https://doi.org/10.1054/bjoc.1999.1002
39. Kimpara K, Shinichi A, Maki
T, Yuichi T. Working Memory Impacts Endurance for Dyspnea. Physiotherapists, Eur Respir J 2020;56:2455.
https://doi.org/10.1183/13993003.congress-2020.2455.
40. Williams MT, John D, Frith P.
Comparison of the DysÂpnoea-12 and Multidimensional Dyspnoea Profile in people
with COPD. Eur Respir J 2017;49:1600773.
https://doi.org/10.1183/13993003.00773-2016.
41. Koskela J, Kupiainen H, Kilpeläinen M, et al. Longitudinal HRQoL shows divergent trends and identifies constant deÂcliners
in asthma and COPD. Respir Med 2014;108:463-71.
https://doi.org/10.1016/j.rmed.2013.12.001
42. Meek PM, Banzett R, Parsall
MB, et al. Reliability and validity of the multidimensional dyspnea profile.
Chest 2012;141:1546-53.
https://doi.org/10.1378/chest.11-1087.
43. Belo LF, Rodrigues A,
Vicentin AP, et al. A breath of fresh air: Validity and reliability of a
Portuguese version of the Multidimensional Dyspnea Profile for patients with
COPD. PLoS One 2019;14:e0215544.
https://doi.org/10.1371/jourÂnal.pone.0215544.
44. Mahler DA, Wells CK.
Evaluation of Clinical Methods for Rating Dyspnea. Chest 1988;93:580-6.
https://doi.org/10.1378/chest.93.3.580
45. Guidance Respiratory
questionnaire and instructions to interviewers (1966)
https://www.ukri.org/publications/respiratory-questionnaire-and-instructions-to-interviewÂers-1966/.
46. Güell R, Casan P, Sangenís M, et al. Traducción
española y validación de un cuestionario de calidad de vida en
paciÂentes con enfermedad pulmonar obstructiva crónica. Arch Bronconeumol 1995;31:202-10.
https://doi.org/10.1016/S0300-2896(15)30925-X
47. Guyatt GH, Berman LB,
Townsend M. Long-term outcome after respiratory rehabilitation. CMAJ 1987;137:1089â95.
48. Guyatt GH, Berman LB,
Townsend M, Pugsley SO, ChamÂbers LW. A measure of quality of
life for clinical trials in chronic lung disease. Thorax 1987;42:773-8. https://doi.org/10.1136/thx.42.10.773.
49. Guyatt GH, Townsend M, Berman
LB, et al. A comparison of Likert and visual analogue scales for measuring
change in function. J Chronic Dis 1987;40:1129-33.
https://doi.org/10.1016/0021-9681(87)90080-4
50. Valero-Moreno S,
Castillo-Corullón S, Prado-Gascó VJ, Pérez-Marín M,
Montoya-Castilla I. Chronic Respiratory Disease Questionnaire (CRQ-SAS):
Analysis of psychoÂmetric properties. Arch Argent Pediatr 2019;117:149-56. https://doi.org/10.5546/aap.2019.eng.149.
51. Guyatt GH, Townsend M, Berman
LB, Pugsley SO. QualÂity of life in patients with chronic
airflow limitation. Br J Dis Chest. 1987;81:45-54. https://doi.org/10.1016/0007-0971(87)90107-0.
52. Cella DF, Bonomi AE, Lloyd
SR, et al. Reliability and validity of the functional assessment of cancer
therapy-lung (FACT-L) quality of life instrument. Lung Cancer 1995;12:199-220. https://doi.org/10.1016/0169-5002(95)00450-F121
53. Groenvold M, Petersen MA,
Aaronson NK, et al. The develÂopment of the EORTC QLQ-C15-PAL: A shortened quesÂtionnaire
for cancer patients in palliative care. Eur J Cancer 2006;42:55-64.
https://doi.org/10.1016/j.ejca.2005.06.022
54. Groenvold M, Petersen MA,
Aaronson NK, et al. EORTC QLQ-C15-PAL: the new standard in the asÂsessment of
health-related quality of life in adÂvanced cancer? Palliat Med 2006;20:59-61.
https://doi.org/10.1191/0269216306pm1090ed
55. Pilz MJ, Aaronson NK, Arraras JI, et al. Evaluating the Thresholds for Clinical Importance of
the EORTC QLQ-C15-PAL in Patients Receiving Palliative Treatment. J Palliat Med 2021;24:397-404.
https://doi.org/10.1089/jpm.2020.0159.
56. Bergman B, Aaronson NK,
Ahmedzai S, et al. The EORTC QLQ-LC13: a modular supplement to the EORTC core
qualÂity of life questionnaire (QLQ-C30) for use in lung cancer clinical
trials. Eur J Cancer 1994;30:635-42.
https://doi.org/10.1016/0959-8049(94)90535-5
57. Nicklasson M, Bergman B.
Validity, reliability and clinical relevance of EORTC QLQ-C30 and LC13 in
patients with chest malignancies in a palliative setting. Qual Life Res 2007;16:1019-28. https://doi.org/10.1007/s11136-007-9210-8.
58. Bausewein C, Farquhar M,
Booth S, et al. Measurement of breathlessness in advanced disease: A systematic
review. Respir Med 2007;101:399-410.
59. Lovell N, Etkind SN, Bajwah
S, et al. To What Extent Do the NRS and CRQ Capture Change in Patientsâ
Experience of Breathlessness in Advanced Disease? Findings From a Mixed-Methods
Double-Blind Randomized Feasibility Trial. J Pain Symptom Manage 2019;58:369-81.e7. https://doi.org/10.1016/j.jpainsymman.2019.06.004
60. Bourke SC, McColl E, Shaw PJ,
Gibson GJ. Validation of quality of life instruments in ALS. Amyotroph Lateral
Scler Other Motor Neuron Disord 2004;5:55-60.
https://doi.org/10.1080/14660820310016066.
61. Dorman S, Byrne A, Edwards A.
Which measurement scales should we use to measure breathlessness in palliative
care? A systematic review. Palliat Med 2007;21:177-91. https:// doi.org/10.1177/0269216307076398.
62. Booth S, Adams L. The shuttle
walking test: a reproducÂible method for evaluating the impact of shortness of
breath on functional capacity in patients with advanced cancer. Thorax 2001;56:146-50. https://doi.org/10.1136/thorax.56.2.146.
63. Wilcock A, Crosby V, Clarke
D, et al. Reading numbers aloud: a measure of the limiting effect of
breathlessness in patients with cancer. Thorax 1999;54:1099-103.
https://doi.org/10.1136/thx.54.12.1099
64. Wilcock A, Walker G,
Manderson C, et al. Use of Upper Limb Exercise to Assess Breathlessness in
Patients with Cancer: Tolerability, Repeatability, and Sensitivity. J Pain
Symptom Manage 2005;29:559-64.
https://doi.org/10.1016/j.jpainsymman.2004.09.003
65. Mori M, Yamaguchi T, Matsuda Y, et al. Unanswered questions and future direction in the
management of terminal breathlessness in patients with cancer. ESMO Open 2020;5 Suppl 1: e000603.
https://doi.org/10.1136/esmoopen-2019-000603.
66. Koller M, Warncke S,
Hjermstad MJ, et al. European OrgaÂnization for Research and Treatment of
Cancer (EORTC) Quality of Life Group; EORTC Lung Cancer Group. Use of the lung
cancer-specific Quality of Life Questionnaire EORTC QLQ-LC13 in clinical
trials: A systematic review of the literature 20 years after its development.
Cancer 2015;15;121:4300-23.
https://doi.org/10.1002/cncr.29682.
67. Campbell ML. Psychometric
testing of a respiratory distress observation scale. J Palliat Med 2008;11:44-50. https://doi.org/10.1089/jpm.2007.0090.
68. Campbell ML, Templin T, Walch
J. A Respiratory Distress Observation Scale for patients unable to self-report
dysÂpnea. J Palliat Med 2010;13:285-90.
https://doi.org/10.1089/jpm.2009.0229.
69. Zhuang Q, Yang GM, Neo SH-S,
et al. Validity, Reliability, and Diagnostic Accuracy of the Respiratory
Distress ObserÂvation Scale for Assessment of Dyspnea in Adult Palliative Care
Patients. J Pain Symptom Manage 2019;57:304-10.
https://doi.org/10.1016/j.jpainsymman.2018.10.506
70. Chesteen K, Kothare N, Huth
H, Misra S. Implementing a Respiratory Distress Scale for Unconscious Patients
in a Palliative Care Unit. J Pain Symptom Manage 2022;63:871.
https://doi.org//10.1016/j.jpainsymman.2022.02.063.
71. Decavèle M, Rivals I,
Persichini R, et al. Prognostic Value of the Intensive Care Respiratory
Distress Observation Scale on ICU Admission. Respir Care 2022;67:823-32.
https://doi. org/10.4187/respcare.09601
72. Decavèle M, Rozenberg
E, Niérat M-C, et al. Respiratory distress observation scales to predict
weaning outcome. Crit Care 2022;26:162.
https://doi.org/10.1186/s13054-022- 04028-712