Autor : De Vito, Eduardo L.1-2
1 Medical Research Institute Alfredo Lanari, Faculty of Medicine, University of Buenos Aires, Buenos Aires, Argentina. 1Centro del Parque, Respiratory Care Department, Buenos Aires, Argentina.
https://doi.org/10.56538/ramr.OKRA7194
Correspondencia : Eduardo Luis De Vito, eldevito@gmail.com
ABSTRACT
All the theories about the
mechanisms of generation of dyspnea had defenders and detractors and,
interestingly, with the development of sophisticated neurophysiological techniques
and functional imaging, it has been possible to rank each one of them. All have
survived the passage of time and none can singularly explain dyspnea in all
cliniÂcal situations, showing the complex and multifactorial nature of the
phenomenon. The concept of length-tension inappropriateness has found support
in recent decades with new evidence in its favor. Specially
with the discovery of the pathways involved and with the application of
neurophysiological knowledge, the length-tension inappropriateÂness theory
would be refined with the corollary discharge or efferent copy. This corolÂlary
discharge or efferent copy is a basic attribute of the nervous system found in
the animal kingdom, from invertebrates to primates and in the human species.
This article is dedicated to the history of the efferent copy and its
incorporation as a hypothesis to explain dyspnea, which is currently the most
accepted one.
Key words: Dyspnea, Breathing Mechanics, Corollary Discharge, Efferent Copy
RESUMEN
Todas
las teorÃas sobre los mecanismos de generaciÃģn de disnea tuvieron defensores y
detractores e, interesantemente, con el desarrollo de sofisticadas tÃĐcnicas
neurofisiÂolÃģgicas y de imÃĄgenes funcionales ha sido posible jerarquizar cada
uno de ellos. Todas han sobrevivido al paso del tiempo y ninguna puede explicar
por sà sola la disnea en todas las situaciones clÃnicas, lo cual habla de la
naturaleza compleja y multifactorial del fenÃģmeno. El concepto de inadecuaciÃģn
tensiÃģn y longitud hallÃģ en las Últimas dÃĐcadas un sustento con nuevas
evidencias a su favor. En particular, con el hallazgo de las vÃas involucradas
y con la aplicaciÃģn de conocimientos neurofisiolÃģgicos, la teorÃa de la
inadecuaciÃģn tensiÃģn y longitud se verÃa refinada con la descarga corolaria o copia eferente. Esta descarga corolaria o copia eferente es un atributo bÃĄsico del
sistema nervioso, que se encuentra en el reino animal, desde los invertebrados
a los primates y en la especie humana. Este artÃculo estÃĄ dedicado a la
historia de la copia eferente y su incorporaciÃģn como hipÃģtesis para explicar
la disnea, la mÃĄs aceptada en la actualidad.
Palabras
clave: Disnea,
MecÃĄnica respiratoria, Descarga corolaria, Copia
eferente
Received: 11/26/2022
Accepted: 07/31/2023
INTRODUCTION
Why canât we tickle ourselves?
Why doesnât an electric fish electrocute itself? Why doesnât the strong
vibration of a cricketâs legs disturb it? Why donât bats confuse their sounds
with those of others? and ultimately, why do we
experience dyspnea? Because of the efferent copy (EC) or corollary discharge
(CD).1
An EC or CD is a fundamental
attribute of the nervous system found in the animal kingdom, from invertebrates
to primates, and in the human speÂcies.1
This article is dedicated to the history
of the EC and its incorporation as a hypothesis to explain dyspnea, which is
currently the most accepted one.
When the motor system sends a
signal to a muscle, it also sends an internal copy of the sigÂnal that does not
exit the central nervous system (CNS). This internal signal is called the
âefferent copyâ or âcorollary dischargeâ. This EC or CD is compared with the
sensory input or reafferent, which comes from the
moving muscle. If the EC/ CD and the reafferent are
equal, it means that the intended movement is the same as the executed
movement. This prevents unnecessary self-induced perceptions (Figure 1).
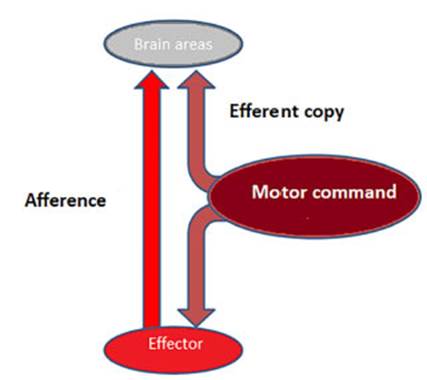
The command sent from a motor region
of the central nervous system (motor command) is copÂied and sent to other
regions of the CNS before the movement occurs. Subsequently, the effector
(e.g., muscle) sends afferent information to the CNS, where both signals are
compared. If both signals are equal (intended movement = executed movement),
there are no unnecessary self-induced perceptions.
HISTORY OF THE EFFERENT COPY
The first to propose the
existence of the EC was Hermann von Helmholtz in the mid-19th century: the
CNS needed to create an EC from the motor command that controls the eye muscles
in order to help the brain determine the location of an object in relation to
the head. He coined the term âpsychoÂphysicsâ and established a precise and
non-linear relationship between the magnitude of physical stimuli and the
perceived intensity. Helmholtz paved the way for the development of the psychoÂphysical
laws of Weber and Stevens.
The initial concept of EC was
disregarded for 75 years after Sir Charles Scott Sherrington (NoÂbel Prize in
Medicine in 1932) strongly criticized Hermann von Helmholtzâs ideas in 1920.2 It was not
until the mid-20th century that Erich von Holst and Horst Mittelstaedt,
in 1950, described the principle of reafference to
explain how an orÂganism is able to distinguish between a reafferent
(self-generated) sensory stimulus and an exafferÂent
(externally generated) stimulus.3 This concept
contributed to the understanding of interactive processes between the CNS and
its periphery and received a total of 2973 citations.
It was Roger Wolcott Sperry
(Nobel Prize in Physiology in 1981) who, thanks to his research on the optokinetic nystagmus reflex,
introduced the concept of the CD and is considered the creator of that term.4 His article
has been cited 1636 times. The EC has been implicated in the lack of dyspnea in
patients with COVID-19,5 a hypothesis that deserves to be explored in
greater detail.
Differences between efferent copy and corollary discharge
Through different experimental
lines, Von Holst and Mittelstaedt3 primarily
referred to the concept of âefferent copyâ while their contemporary SperÂry4 coined the
concept of âcorollary dischargeâ. The first concept involves a real copy of the
motor command (the efference) directed to the
muscles. This term seemed appropriate for the questions that Von Holtz and Mittelstaedt addressed in inÂvertebrates and for the
general analysis of sensory processing that takes place in relation to motor
discharge. However, it has become evident that the connection between motor and
sensory areas can be produced at several levels of motor control.
Through studies on fish, Sperry4 used the
second concept, âcorollary dischargeâ to denote motor sigÂnals that influence
sensory processing. However, his conception was less specific as regards the
location where the motor discharge to the sensory pathways should arise. So,
the terms have a differÂent history and some differences regarding the level of
complexity, but they are often used interchangeÂably. For the purposes of
this article, they will be mentioned interchangeably.
In the coming decades, the
concept of âefferÂent copyâ will expand significantly. Poulet
et al suggested the use of CD as a broad concept to encompass neural signals
generated in motor cenÂters that are not directly used to generate ongoing
motor activity but often act to modulate sensory processing.6
TAXONOMIC CLASSIFICATION OF EFFERENT COPIES OR COROLLARY DISCHARGE
How is sensory
processing connected in inverteÂbrates and dyspnea in humans? What taxonomic
type of internal copy produces dyspnea? Crapse and Sommer suggested a functional taxonomic classifiÂcation of
efferent copies for the entire animal kingÂdom.1
Corollary discharge can be globally classified into categories of
lower and higher order based on the function and operational impact of the
signal.
The lower-order signaling
is ubiquitous, as it is necessary for any animal equipped with sensory and
motor systems. In this context, corÂollary discharge is a discriminatory mechanism
that prevents maladaptive responses and sensory overload by restricting or
filtering information. The cricket doesnât stun itself (and it can hear other
environmental noises), and the electric fish doesnât electrocute itself.
When Titi
monkeys howl, they face the same problem as crickets: initially, the sounds
they emit should affect their hearing. A protective mechaÂnism can be observed
in the primary auditory cortex of the Titi monkey,
where many neurons are suppressed during self-vocalization. SuppresÂsion begins
about 200 ms before vocalization and continues
throughout its duration. This could be a case where the CD interconnects motor
and sensory areas that occupy comparable spaces of a sensorimotor pathway.1
Higher-order signaling plays a role in two types of functions. On the perceptual side,
it facilitates the contextual interpretation of sensory information (analysis)
and the construction and maintenance of an internal representation of this
information (stability). On the sensorimotor side, it facilitates the
acquisition of new motor patterns (learning) and the execution of sequences of
rapid movements (planning). This type of corolÂlary discharge allows specific
brain structures to make appropriate adjustments in anticipation of the sensory
input. Each bat only hears its own sound and not the sound of others, enabling
them to build a cohesive representation of the world. So far, the
higher-order CD has only been identified in vertebrates.
There isnât a single type of CD,
but rather numerous subtypes that correspond to both the anatomical levels of
the source and the target, as well as functional utilities.1
As can be observed, this taxonomy illustrates the crucial point
that, although Sperryâs original concept4
of CD aligns with the general flow of information from motor
systems to sensory systems throughout the animal kingdom, it appears
inappropriately simplistic to use a single concept to describe the signal.
IDENTIFICATION OF COROLLARY DISCHARGE PATHWAYS
The neurons mediating these
signals have been hard to identify. The first evidence came from a single multisegmental interneuron of CD responÂsible for
presynaptic and postsynaptic inhibition of auditory neurons in cricket singing
(Gryllus biÂmaculatus).6 Similar structures were found in the tadpole, the river
crab, and the sea slug Aplysia. Studies in
these species contribute to the classiÂcal understanding of CD: they project
and target regions involved in the processing of reafferent
information.
However, sensory processing is highly
dynamic, taking into account the behavioral state of the animal. Therefore,
analyzing sensory pathways in preparations under anesthesia or at rest may not
provide a complete picture of sensory processing. Perhaps, in evolutionary
terms, the CD initially modulated real activity, and then, in more complex
brains, also targeted the regions involved in sensoÂrimotor integration or
motor planning.7
Our muscles are sensitive; this
includes the respiratory muscles. In other words, we receive sensory signals
from muscles that reach our consciousness and inform us about what is hapÂpening
in those muscles, similar to how sensory signals from the skin tell us what is
happening there. Studies in animals showed that a copy of the respiratory
motor impulse is transmitted to the midbrain and thalamus.1-8
DYSPNEA AND COROLLARY DISCHARGE
In 1978, with an article that got
more than 1000 citations, McClosky et al suggested
that corollary discharge signals, or ECs
originating from the respiratory centers in the brainstem can be transÂmitted
to higher brain centers and give rise to a conscious awareness of the output
motor command. This may play a significant role in the formation of the
sensation of dyspnea.11
The concept of CD is the most
widely accepted to explain the origin of the sensation of inspiratory effort
and dyspnea.12-14 The proposed scheme for the respiratory system is
essentially the same as the one described in Figure 1. Unlike pain recepÂtors,
the afferents projecting to the higher brain centers to compare with the EC are
diverse.15 Additionally, the respiratory system has an autoÂmatic
(brainstem) and voluntary (motor cortex) motor command. This CD from different
sources most likely gives rise to different sensations.15
Therefore, in our opinion, dyspnea is not merely a carbon copy of
pain.
Figure 2 depicts the CD in the
respiratory system, with its dual involuntary and voluntary innervation. During
involuntary breathing, respiÂratory centers send an EC to the sensory cortex,
whereas during voluntary respiratory efforts, it is the motor cortex that sends
the copy. SimultaneÂously, the respiratory muscles send afferents to the
sensory cortex. When there is proper corresponÂdence between the motor command
and incoming afferent information from sensory receptors, there shouldnât be
any sensation of dyspnea (Figure 2). On the contrary, when there is no
correspondence, the resulting neuromechanical
uncoupling conÂtributes to the genesis of dyspnea. This exchange between the
motor command and the sensory cortex is currently the most accepted mechanism
by which awareness of respiratory effort is achieved.
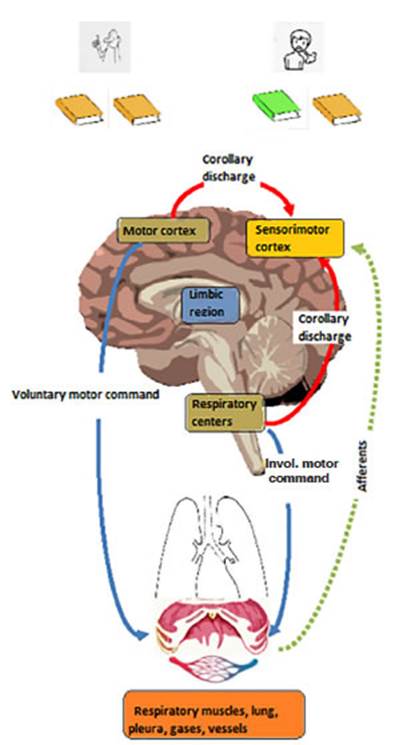
If both copies (efferent and reafferent) are equivalent (same color), there is no
dyspnea; if the copies differ (different color), dyspnea occurs.
Why is our breathing not usually self-perceived?
The sensory cortex also receives
afferents from events that occur in the chest and respiratory muscles and
processes the information.14 When it receives the EC, the sensory cortex adjusts acÂcordingly
to minimize, eliminate, or compensate for the sensory consequences of movement.
Due to this general strategy, breathing under normal conditions is an
unconscious process1 (and within
certain ventilation limits).
What is the relationship between the length-tension inappropriateness of
Campbell and the efferent copy?
In fact, a dissociation between
the motor command and the mechanical response of the respiratory system recalls
the theory of length-tension inapÂpropriateness by Campbell and Howell in the
1960s.15 The theory
has been generalized to include not only information arising in the respiratory
muscles but also the one emanating from receptors throughout the entire
respiratory system, and has been named with various terms.15-19
â Neuromechanical
dissociation.
â Efferent-reafferent
dissociation.
â Length-tension
inappropriateness.
â Neuroventilatory
dissociation.
â Afferent discordance
(mismatch).
â Neuromechanical
decoupling.
â Neuromuscular dissociation.
What respiratory conditions can have a dissociation
between efferent and afferent information and result in dyspnea?
In addition to the mentioned
neurophysiological findings, various experimental data and clinical
observations are consistent with the concept of efferent-afferent
inappropriateness.20-25
In both patients and healthy
individuals, temporary suppression of ventilation during speech or eating causes
a mismatch between the respiratory motor command and the expected movement.
When normal subjects breathe CO2, their
ventilation increases, and most experience dysÂpnea. However, if ventilation is
reduced while CO2 remains constant,
subjects report a marked increase in the intensity of breathlessness, even
though the chemical drive to breathe has not changed.
When normal subjects are forced
to breathe at an inspiratory flow that is different from what they have chosen
as the most comfortable, they experience a sensation of air hunger.
A similar phenomenon can occur in
patients receiving mechanical ventilation and showing unsuitability for the
respirator.
All of this suggests that, under
a given set of conditions, the brain âexpectsâ a certain ventilaÂtion pattern
and associated afferent feedback, and deviations from this pattern cause or
intensify the sensation of dyspnea.
Is there any relationship between corollary discharge and the lack of
dyspnea in patients with COVID-19?
COVID-19 is surprising and
intriguing in various aspects. One of its relevant characterÂistics is the
ability to recognize the absence of dyspnea in the majority of cases. If the physiopathological mechanisms of dyspnea developÂment are
not yet well understood, we shouldnât be surprised, since we have limited
knowledge of the dyspnea mechanisms in COVID-19.5
In some series, intubated and ventilated subjects exhibited
tachypnea and tachycardia.26-28 In a
retrospective study, dyspnea and chest tightness were much more common in
deceased patients.29 Dyspnea was
one of the associated predictors of severe illness and death.30
To understand the absence of dyspnea in COVID-19, the main focus
is on phenotypes that show severe hypoxia and almost normal respiratory system distensibility. In respiratory distress due to COVID-19
pneuÂmonia, the respiratory system (transpulmonary distensibility and driving pressure) was reported to be
pseudonormal.31,
32 From
a physiopathoÂlogical perspective, which does not
exclude the direct neurotoxic effect of the virus and a systemic response in
the infectious context but rather encompasses it, the lack of dyspnea in
COVID-19 can be explained by an adaptation in the sensory cortex of the brain
of the two signals coming from the motor command and the periphery via CD.5
CONCLUSIONS AND THERAPEUTIC PROJECTIONS
All animals, from the humble
nematode to the cognitively advanced primate require the type of signaling that
allows the CD which protects against unnecessary self-induced perceptions. We
are still in an embryonic stage of CD research in the animal kingdom. However, the
exchange between the motor command and the sensory corÂtex is currently the
most accepted mechanism by which awareness of respiratory effort is achieved. Neuronal
pathways have been identified. Indeed, a dissociation
between the motor command and the mechanical response of the respiratory sysÂtem
recalls the Campbell and Howellâs âlength-tension inappropriatenessâ theory
from the 1960s. The future goal will be to discover how the CD influences
perception. Experiments so far have shown that inactivation of CD pathways can
alter behavior, and subtle perceptual changes may justify these behavioral
alterations. This knowledge may be highly relevant for the relief of refractory
dyspnea.
KEY POINTS
âĒ Clinical,
experimental, neurophysiological data, and clinical observations support the concept of a lack of tension-length adequacy, neuroÂmechanical dissociation, or efferent-reafferent dissociation (EC/CD) as the central core in the
genesis of dyspnea.
âĒ The central concept is that,
under a given set of conditions, the brain âexpectsâ a certain ventilaÂtion
pattern and associated afferent feedback; deviations from this pattern cause or
intensify the sensation of dyspnea.
âĒ It is necessary to go deeper
into the role of CD in the absence of dyspnea in COVID-19 as well as in other
conditions.
REFERENCES
1. Crapse
TB, Sommer MA. Corollary discharge
across the animal kingdom. Nat Rev Neurosci
2008;9:587-600. https://doi.org/10.1038/nrn2457
2. Efference
copy [Internet]. Available from:
https://en.wikipedia.org/w/index.php?title=Efference_copy&oldid=841428446).
3. Von Holst E, Mittelstaedt H. Das Reafferenzprinzip:
Wechselwirkungen zwischen Zentralnervensystem und Peripherie.
Sci Nat 1950;37:464-76.
https://doi.org/10.1007/BF00622503
4. Sperry RW. Neural
basis of the spontaneous optokinetic response
produced by visual inversion.J Comp Physiol Psychol 1950;43:482-9. https://doi.org/10.1037/h0055479
5.
De Vito EL. Possible Role of Corollary Discharge in Lack of
Dyspnea in Patients With COVID-19 Disease [Internet].
Front Physiol 2021;12.
http://dx.doi.org/10.3389/fphys.2021.719166
6. Poulet
JF, Hedwig B. The cellular basis of a corollary disÂcharge.
Science. 2006;311: 518-22.
http://dx.doi.org/10.1126/science.1120847.
7. Poulet
JF, Hedwig B. New insights into corollary discharges mediated by identified
neural pathways. Trends Neurosci 2007;30:14-21. https://doi.org/10.1016/j.tins.2006.11.005
8. Chen Z, Eldridge FL, Wagner
PG. Respiratory-associated rhythmic firing of midbrain neurones
in cats: relation to level of respiratory drive [Internet]. The Journal of
Physiology 1991;437:305-25.
http://dx.doi.org/10.1113/jphysiol.1991.sp018597
9. Chen Z, Eldridge FL, Wagner
PG. Respiratory-associated thalamic activity is related to level of respiratory
drive [Internet]. Respiration Physiology 1992; 90:99-113.
http://dx.doi.org/10.1016/0034-5687(92)90137-l
10. Matthews PBC. Where Does
Sherringtonâs âMuscular Senseâ Originate? Muscles, Joints, Corollary
Discharges? [Internet]. Annual
Review of Neuroscience 1982; 5: 189-218.
http://dx.doi.org/10.1146/annurev.ne.05.030182.001201
11. McCloskey DI. Kinesthetic sensibility. Physiol
Rev 1978;58:763-820.
https://doi.org/10.1152/physrev.1978.58.4.763
12. Spengler CM, Banzett RB, Systrom DM, Shannon
DC, Shea SA. Respiratory sensations during heavy exercise in
subjects without respiratory chemosensitivity.
Respir Physiol 1998;114:65-74. https://doi.org/10.1016/S0034-5687(98)00073-5
13. Booth S, Dudgeon D. Dyspnoea in Advanced Disease: A Guide to Clinical
Management. Oxford University Press; 2006. 271 p.
https://doi.org/10.1093/acprof:oso/9780198530039.001.0001
14. Fukushi
I, Pokorski M, Okada Y. Mechanisms underlying the
sensation of dyspnea. Respir Investig
2021;59:66-80.
https://doi.org/10.1016/j.resinv.2020.10.007
15. Campbell EJ, Howell JB. The sensation of breathlessness. Br Med Bull 1963;19:36-40.
https://doi.org/10.1093/oxfordjournals.bmb.a070002
16. Parshall
MB, Schwartzstein RM, Adams L, et al. An OfÂficial
American Thoracic Society Statement: Update on the Mechanisms, Assessment, and
Management of Dyspnea. Am J Respir Crit Care Med 2012;185:435-52.
https://doi.org/10.1164/rccm.201111-2042ST
17. OâDonnell DE, Webb KA. Exertional
breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. Am Rev Respir
Dis 1993;148:1351-7.
https://doi.org/10.1164/ajrccm/148.5.1351
18. Banzett
RB, Lansing RW, Brown R, Topulos GP, Yager D, Steele SM, et al. âAir hungerâ from increased PCO2 persists
after complete neuromuscular block in humans. Respir Physiol 1990;81:1-17.
https://doi.org/10.1016/0034-5687(90)90065-7
19. Nishino T. Dyspnoea: underlying mechanisms and treatment. Br J Anaesth 2011;106: 463-74.
https://doi.org/10.1093/bja/aer040
20. Manning HL, Molinary EJ, Leiter JC. Effect of inspiratory flow rate on respiratory sensation and
pattern of breathing. Am J Respir
Crit Care Med 1995; 151: 751-7.
https://doi.org/10.1164/ajrccm/151.3_Pt_1.751
21. Manning HL, Schwartzstein RM. Pathophysiology of DysÂpnea [Internet]. N
Engl J Med 1995;333:1547-53.
http://dx.doi.org/10.1056/nejm199512073332307
22. Chonan
T, Mulholland MB, Cherniack NS, Altose
MD. EfÂfects of voluntary constraining of thoracic
displacement during hypercapnia. J Appl Physiol 1987;63:1822-8. https://doi.org/10.1152/jappl.1987.63.5.1822
23. Schwartzstein
RM, Simon PM, Weiss JW, Fencl V, WeinÂberger SE.
Breathlessness induced by dissociation beÂtween ventilation and chemical drive.
Am Rev Respir Dis 1989;139:1231-7.
https://doi.org/10.1164/ajrccm/139.5.1231
24. Manning HL, Shea SA, Schwartzstein RM, Lansing RW, Brown R, Banzett
RB. Reduced tidal volume increases âair hungerâ at fixed PCO2 in ventilated quadriplegics. Respir Physiol 1992;90:19-30. https://doi.org/10.1016/0034-5687(92)90131-F
25. Schwartzstein
RM, Manning HL, Woodrow Weiss J, WeinÂberger SE. Dyspnea: A sensory experience
[Internet]. Lung 1990; 168: 185-99. Available from:
http://dx.doi.org/10.1007/bf02719692
26.
Al-Omari A, Alhuqbani WN, Zaidi ARZ, et al. Clinical charÂacteristics
of non-intensive care unit COVID-19 patients in Saudi Arabia: A descriptive
cross-sectional study. J Infect Public Health 2020; 13: 1639-44.
https://doi.org/10.1016/j.jiph.2020.09.003
27.
Guan WJ, Ni ZY, Hu Y, et
al. Clinical Characteristics of Coronavirus Disease 2019
in China. N Engl J Med 2020;382:1708-20.
https://doi.org/10.1056/NEJMoa2002032
28. Li YC, Bai
WZ, Hashikawa T. The neuroinvasive
potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19
patients. J Med Virol 2020;92:552-5.
https://doi.org/10.1002/jmv.25728
29. Chen T, Wu D, Chen H, et al.
Clinical characteristics of 113 deceased patients with coronavirus disease
2019: retrospective study. BMJ 2020;368:m1091.
https://doi.org/10.1136/bmj.m1091
30. Kaeuffer
C, Le Hyaric C, Fabacher T,
et al. Clinical charÂacteristics and risk factors associated with severe COÂVID-19:
prospective analysis of 1,045 hospitalised cases in
North-Eastern France, March 2020. Euro Surveill
[Internet] 2020;25(48).
http://dx.doi.org/10.2807/1560-7917.ES.2020.25.48.2000895
31. Bhatraju PK, Ghassemieh BJ,
Nichols M, et al. Covid-19 in Critically Ill Patients in the Seattle Region -
Case Series. N Engl
J Med. 2020;382:2012-22.
http://dx.doi.org/10.1056/NEJMoa2004500.
32. Viola L, Russo E, Benni M, et al. Lung mechanics in type L CoVID-19
pneumonia: a pseudo-normal ARDS. Transl Med Commun 2020;5:27.
https://doi.org/10.1186/s41231-020-00076-9