Autor : Merine MarĂa Antonela1, Rojas Llanos Georgina1, Gonzalez, Alejandra1
1Pulmonology Service, Hospital Nacional Profesor Alejandro Posadas, Buenos Aires, Argentina
https://doi.org/10.56538/ramr.WGRJ4623
Correspondencia : MarĂa Antonela Merine. E-mail: antomerine@hotmail.com
ABSTRACT
Antiphospholipid syndrome (APS) is an entity characterized by thrombotic phenomena
(arterial and/or venous), fetal losses, and persistent increase in the serum
level of antiphospholipid antibodies.
The most common pulmonary
complications of APS are pulmonary thromboembolism and thromboembolic pulmonary
hypertension. Alveolar hemorrhage is a rare, potentially life-threatening
manifestation (catastrophic APS). The diagnosis is confirmed when more than 20
% of the macrophages in a sample taken by bronchoalveolar
lavage (BAL) are positive for hemosiderin. Radiographic findings most commonly
show ground-glass opacities or consolidations that are usually diffuse,
bilateral, more central than peripheral. The DLCO is
another diagnostic method, with values above 120 % of the predicted
value.
We present the case of a patient
with APS with a history of deep vein thrombosis (DVT) and anticoagulated
pulmonary thromboembolism (PTE), who was admitted with a diagÂnosis of alveolar
hemorrhage (AH).
Key word: Primary antiphospholipid syndrome, Alveolar
hemorrhage
RESUMEN
El
sĂndrome antifosfolĂpido (SAF) es una entidad
caracterizada por fenĂłmenos tromÂbĂłticos (arteriales
y/o venosos), pĂ©rdidas fetales y elevaciĂłn sĂ©rica persistente de anticuerpos antifosfolĂpidos.
Las
complicaciones pulmonares más frecuentes del SAF son: tromboembolismo
pulmonar, hipertensiĂłn pulmonar tromboembĂłlica. La
hemorragia alveolar es una manifestaciĂłn infrecuente y potencialmente mortal
(SAF catastrĂłfico). El diagnĂłstico se confirma cuando en una muestra tomada
mediante lavado bronquioalveolar (BAL), más del 20 %
de los macrĂłfagos son positivos para hemosiderina. Los hallazgos radiográfiÂcos
más comúnmente muestran opacidades en vidrio deslustrado o de consolidación que
suelen ser difusas, bilaterales más centrales que periféricas. La DLCO es otro
método diagnóstico, con valores por encima del 120 % del valor predicho.
Presentamos
el caso de un paciente con SAF con antecedentes de trombosis venosa profunda
(TVP) y tromboembolismo pulmonar (TEP) anticoagulado, que ingresa con diagnĂłstico de hemorragia
alveolar (HA).
Palabras
clave: SĂndrome
antifosfolipido primario, Hemorragia alveolar
Received: 07/24/2023
Accepted: 08/30/2023
INTRODUCTION
Antiphospholipid syndrome (APS) is systemic autoimmune entity characterized by
thrombotic phenomena (arterial and/or venous), fetal losses, and persistent
increase in the serum level of anÂtiphospholipid
antibodies.
Although the global prevalence is
unknown, it is a rare condition, and it is estimated to be present in
approximately 1 % of the general population. It can be primary or in the
context of an underlying disease, usually systemic lupus erythematosus
(SLE) or other systemic autoimmune disorders.
The most common pulmonary complications
include: pulmonary thromboembolism, thromÂboembolic and non-thromboembolic
pulmonary hypertension, microvascular thrombosis,
acute respiratory distress syndrome, and alveolar hemorÂrhage, the latter being
an uncommon and potenÂtially life-threatening manifestation.
CASE REPORT
A 16-year old male consults for a
1-year history of episodes of intermittent hemoptysis. Medical reÂcord: Primary
APS, anticoagulated with acenocouÂmarol
for DVT and PTE, hospitalized for idiopathic thrombocytopenic purpura (ITP) (treated with gammaglobulin)
and alveolar hemorrhage (AH) in February 2021, requiring invasive ventilation for 9 days.
Reason for hospitalization: hemoptysis. Upon admission, the patient was hemodynamically stable; oxygen saturation at 98 %. Presumptive diagnosis of AH.
The following tests were
performed: LaboraÂtory:
normal complement levels, negative ANCA antibodies, negative ANA (antinuclear
antibodies), ESR (erythrocyte sedimentation rate) 23, negative anti-MPO
antibodies, negative anti-PR3 antibodÂies, negative anti-glomerular basement
membrane antibodies. CHEST
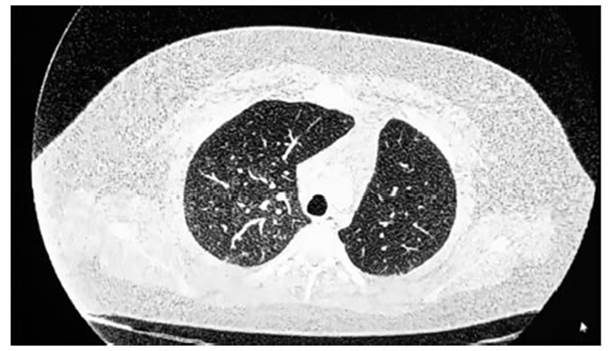
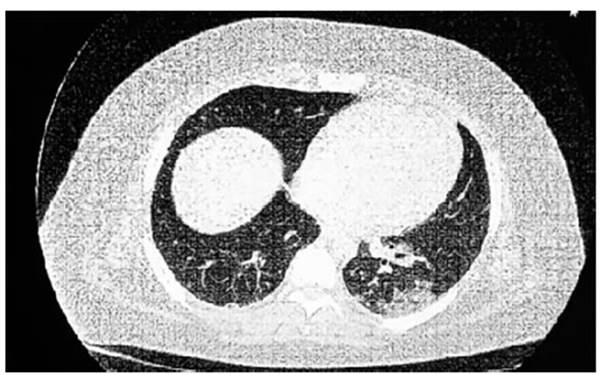
CT SCAN: Increased lung density with ground-glass opacity of
diffuse pulÂmonary infiltration predominantly peribronchoÂvascular
in both lung fields. Consolidation area in the left base (Figure
1 and Figure 2). RFE
+ DIFUSSION: FVC 4.41 (81 %) FEV1 3.31 (73 %) FEV1/FVC
75 %. DLCO 141 % DL/AV: 186 % AV: 75 %. BRONCHOSCOPY: No endoluminal
lesions. Bronchoalveolar lavage (BAL) in basal segments of the left lower lobe with
negative cultures and cytology test showing 95 % siderophages.
Oral anÂticoagulation is discontinued, intravenous heparin is administered,
cultures are taken, and antibiotic therapy is initiated. The patient responds
favorÂably and is then discharged.
The diagnosis of AH in the
context of APS is confirmed based on the CT scan infiltrates, the elevated DLCO
(diffusing capacity of the lungs for carbon monoxide), and a high percentage of
siderophages in the BAL. High-dose corticosteÂroid
immunosuppression is initiated. No further episodes of hemoptysis.
DISCUSSION
The main form of pulmonary
involvement in APS is pulmonary thromboembolism, with a frequency of 3.5-14.1
%. Chronic thromboembolic pulmonary hypertension (CTEPH) is a relatively rare
compliÂcation. Poli et al reported the incidence of
CTEPH after a first episode of pulmonary embolism (PE) to be 0.4 % in their
series, which included 239 patients with PE.1
In a prospective long-term
follow-up study, the cumulative incidence of CTEPH in patients with first-time
diagnosed PE was found to be 11.2 % at 3 months, 12.7 % at 1 year, 13.4 % at 2
years, and 14.5 % at 3 years.2
In the study by S. Sarinc Ulasli et al, which
included 67 patients, acute pulmonary thromÂboembolism (PTE) was detected in 11
patients (16.4 %), and alveolar hemorrhage in 2 (3 %). Four patients with acute
PTE (36 %) developed chronic thromboembolic pulmonary hypertension. One patient
developed CTEPH and diffuse alveolar hemorrhage after
acute PE during follow-up.3
Alveolar hemorrhage (AH) is a
rare and poÂtentially life-threatening condition in APS with a prevalence of
less than 0.7 %. Hillerdal et al reported the first
patient in 1991, and since then, approximately 100 cases have been published.4
In the series by Stoots et al, diffuse alveolar hemorrhage was the initial
presentation of APS in 9/79 patients (11 %), and three
out of 17 patients in a case series were diagnosed with APS only after
presenting DAH. However, two other reviews of 18 and 13 patients observed a
median onset of DAH of 5.9 and 5.8 years, respectively, after the diagnosis of
APS. Additionally, many patients experienced diagnostic delay.5
The risk factors predisposing
patients to alveoÂlar hemorrhage are not well known. Microvascular
thrombosis and rupture of small pulmonary vessels are suggested as potential
pathogenic mechanisms for alveolar hemorrhage in APS. It has been reÂported
that viral infections of the upper respiratory tract or bacterial pneumonia can
trigger episodes of alveolar hemorrhage.6
The management of anticoagulation
in APS patients with alveolar hemorrhage is complex, because discontinuing the
anticoagulation entails a high risk of recurrent thrombosis. It is suggested to
suspend anticoagulation during alveolar hemÂorrhage, to be restarted once it is
under control.7
Corticosteroids induce remission
in most paÂtients; however, almost half of them experience recurrence and
require a steroid-sparing imÂmunosuppressant to maintain remission. Regimens
based on cyclophosphamide or rituximab achieve the highest remission rates (50
%); other strategies include intravenous immunoglobulin, plasmapheresis,
mycophenolate mofetil,
and/or azathioprine.
The work of Cartin-Ceba
et al states that no firm recommendations can be made for preferred
immunosuppressive medications; cyclophosphaÂmide or rituximab were the most
commonly used immunosuppressive agents.8
CONCLUSIONS
AH is an
rare presentation of APS. Bronchoscopy, BAL, DLCO, and chest CT are used to
confirm the diagnosis and help discard other causes of alveolar hemorrhage. The
pulmonary biopsy is the gold standard for confirming
the diagnosis, although the histological pattern is not specific and is not
routinely recommended.
Conflict of interest
The authors have no conflicts of
interest to declare.
REFERENCES
1. Yachoui
R, Sehgal R, Amlani B,
Goldberg JW. AntiphosÂpholipid antibodies-associated diffuse alveolar hemorÂrhage. Semin Arthritis
Rheum. 2015;44:652-7. https://doi.org/10.1016/j.semarthrit.2014.10.013.
2. Garcia, D, Erkan,
D. Diagnosis and Management of the Antiphospholipid
Syndrome. N Engl J Med 2018;378:2010-
21. https://doi.org/10.1056/NEJMra1705454
3. Sarinc
Ulasli, S, Koksal, D, Karcioglu, O. et al. Pulmonary manifestations of antiphospholipid syndrome: a retrospective analysis of 67
patients. J Thromb Thrombolysis.
2021;52:640–5.
https://doi.org/10.1007/s11239-020-02351-w
4. Deane K, West S. Antiphospholipid Antibodies as a Cause of Pulmonary Capillaritis and Diffuse Alveolar Hemorrhage: A Case Series
and Literature Review, Seminars in Arthritis and Rheumatism. 2005;35:154-65. https://doi.org/10.1016/j.semarthrit.2005.05.006.
5. Stoots,
SA, Lief, L, Erkan, D.
Clinical Insights into Diffuse Alveolar Hemorrhage in Antiphospholipid
Syndrome. Curr Rheumatol
Rep. 2019;21:56. https://doi.org/10.1007/s11926-019-0852-7
6. Seth I, Bhagavata
Srinivasan SP, et al. Diffuse alveolar haemorrhage as a rare complication of antiphosphoÂlipid
syndrome. Respirology Case Reports. 2022;10:e0948.
https://doi.org/10.1002/rcr2.948.
7. Miyakis
S, Lockshin MD, Atsumi T,
et al. International consensus statement on an update of the classification
criteria for definite antiphospholipid syndrome
(APS). J Thromb Haemost.
2006;4:295-306. https://doi.org/10.1111/j.1538-7836.2006.01753.x
8. Cartin-Ceba
R, Peikert T, Ashrani A, et
al. Primary antiphospholipid syndrome-associated
diffuse alveolar hemÂorrhage. Arthritis Care Res (Hoboken).
2014;66:301-10. https://doi:10.1002/acr.22109. PMID:
23983016.