Autor : De Vito, Eduardo L1-2, Arce, Santiago C1, Monteiro, Sergio G1
1 Medical Research Institute Alfredo Lanari, Faculty of Medicine, University of Buenos Aires, Buenos Aires, Argentina. 2Centro del Parque, Respiratory Care Department, Buenos Aires, Argentina.
https://doi.org/10.56538/ramr.QVT9846
Correspondencia : Eduardo Luis De Vito, eldevito@gmail.com
ABSTRACT
This article is devoted to a
detailed analysis of the mechanisms of dyspnea. Chemical control of breathing,
neural reflexes, breathing mechanics, the cost of oxygen to breathe, and the
mismatch between tension and muscle fiber length will be discussed. In general,
the different explanations were associated with the development of devices and
study methodologies in pulmonary laboratories. All the theories had defenders
and detractors and, interestingly, with the development of sophisticated
neurophysiological techniques and functional imaging, it has been possible to
prioritize each of the mechanisms. All have survived the passage of time and
none can singularly explain dyspnea in all clinical situations, showing the
complex and multifactorial nature of the phenomenon.
Key words: Dyspnea, Physiology, Physiopathology, Breathing mechanics
RESUMEN
Este
artĂculo está dedicado al análisis detallado de los mecanismos de disnea. Se
tratarán el control quĂmico de la respiraciĂłn, los reflejos neurales, la
mecánica resÂpiratoria, el costo de oxĂgeno para respirar y la inadecuaciĂłn
entre tensiĂłn y longitud de la fibra muscular. En general, las diferentes
explicaciones estuvieron asociadas al desarrollo de aparatos y metodologĂas de
estudio de los laboratorios pulmonares. Todas las teorĂas tuvieron defensores y
detractores e, interesantemente, con el desarrollo de sofisticadas técnicas
neurofisiológicas y de imágenes funcionales ha sido posible jerarquizar cada
uno de los mecanismos. Todas han sobrevivido al paso del tiempo y ninguna puede
explicar de manera unicista la disnea en todas las
situaciones clĂnicas, lo cual habla de la naturaleza compleja y multifactorial
del fenĂłmeno.
Palabras
clave: Disnea,
FisiologĂa, FisiopatologĂa, Mecánica respiratoria
Received: 11/26/2022
Accepted: 05/09/2023
INTRODUCTION
The first part of this series
analyzes the evolution of the definitions of the term “dyspnea” and the proposed
mechanisms for its generation. It was also mentioned that the experience of
dyspnea is beginning to be seen as a multidimensional phenomenon that
should be centered on what the patient feels. This fact cannot be overlooked,
not even in the presence of the exciting complexÂity of the physiopathological
mechanisms we will analyze.
The physiopathological
mechanisms that exÂplain dyspnea, unlike pain, are complex and can coexist; but
depending on the clinical condition, some may be more relevant than others.
However, there are common denominators, and there is one dyspnea-producing
mechanism that is accepted as predominant. The experience of dyspnea involves
both sensory components (intensity and quality) and affective components
(discomfort, distress) that generally impact or impose a burden on an
individual’s ability to perform activities of daily living (quality of life).1
DYSPNEA AND CHEMICAL CONTROL (HYPOXEMIA, HYPERCAPNIA, ACIDOSIS)
In 1868, Pfluger
observed that hypoxemia and hypercapnia produced
dyspnea, but considered hypoxemia to be of greater importance. Eight years
later, Haldane and Smith found that while breathÂing in a closed circuit with
increasing levels of CO2 up to 3% (23
mmHg), the individuals experienced dyspnea, but not until the O2
concentration had dropped to 14%. In 1910, Winterstein
introduced the concept of the H+
ion as a stimulant of ventilaÂtion and a producer of dyspnea.2
These experiments were revisited
in light of the possibility of more reliable gasometric
determinaÂtions.3 The concept of the H+
ion as a stimulant was retained, but something that now seems
quite obvious was defined: dyspnea is very intense with hypercapnic
hypoxia, less intense with hypercapÂnia and hyperoxia, and moderate with hypocapnic
hypoxia.3
This period finished with
Jonathan Meakins’ article in 1923 where he stated
that “dyspnea is usually produced by two causes: the need for oxyÂgen and the
retention of carbon dioxide, relative or absolute”.3
Meakins’ description deserves to be
reproduced: “The use of oxygen, of course, does not eliminate the need to adopt
all other means to treat heart failure, and by no means are physical and mental
rest less important [...]. It is remarkÂable how patients improve with good
nursing care and general comfort”. It is appealing to speculate that with these
words, Meakins anticipated the multidimensional
concept of dyspnea by several decades.
This conceptual framework by Meakins justiÂfied certain clinical observations regarding
the acute effects of inhaling CO2 in normal
subjects or patients:
1. Healthy individuals engaged in
physical activÂity are capable of identifying hypercapnia
due to CO2 inhalation if
they are instructed how to maintain ventilation proportional to their physical
activity (the addition of hypercapnia to exercise
would increase ventilation, and not doing so would lead to more dyspnea).
2. Various studies showed that
patients with chronic poliomyelitis and respiratory failure reported ventilatory discomfort when the PCO2
increased by about 10-20 mmHg.
3. Patients with high cervical
spinal cord injuries who were chronically ventilated were able to detect
increases in PCO2 with a
sensation deÂscribed as “air hunger.”
However, in patients with chronic
obstructive pulmonary disease (COPD) or neuromuscular disorders (NMDs) with
chronic CO2 retention, it
wasn’t clear to what extent hypercapnia was related
to dyspnea:
1. Patients with COPD or NMDs and
chronic hyÂpercapnia may experience little dyspnea at
rest.
2. In other clinical conditions (such
as bronchial asthma), dyspnea can be present with eucapnia
or even hypocapnia.
3. Similarly, there are many
patients with hypoxÂemia who do not experience dyspnea, and vice versa.
Furthermore, some patients show slight improvement when oxygen administration
corÂrects hypoxemia.
Clearly, there were many aspects
to clarify durÂing those times, and it wasn’t until the early 21st century that
it was understood that if the information from chemoreceptors (hypoxia) and
mechanoreceptors indicates inability to adequately respond to the efferent
impulse to the respiratory muscles, dyspnea is produced. Indirect evidence
suggests that hypoxia leads to dyspnea through corollary discharge to higher
centers, if ventilation and PCO2 are limited
to normal levels.4,
5
DYSPNEA AND REFLEXES (INTRAVASCULAR AND MUSCULAR RECEPTORS, VAGUS NERVE)
In 1931, basing on clinical
observations, Cullen et al questioned the explanation that blood chemical changes
were the cause of dyspnea. It was already evident that dyspnea often had little
or nothing to do with impaired gas exchange. Arterial blood gases could be
completely normal and have at the same time considerable dyspnea. These queries
led to the search for new mechanisms. This was the onset of the neural reflex
era.6
In 1932, Harrison et al
demonstrated that breathing is stimulated by reflexes mediated by the vagus nerve, originating from the large cenÂtral vessels
(due to increased pressure from heart failure) and from muscular movements.7
Years later, in 1935, the studies
conducted by Gessel and Moyer defined the role of
reflexes in the control of ventilation and dyspnea.8
It was considered that the effects of various combinations of afferent
impulses (physical and chemical) could largely lead to the rhythmic discharge
of respiraÂtory centers originating from reflex mechanisms. However, on the
other hand, the existence of an automatic discharge center under the influence
of chemical and physical changes of nerve impulses wasn’t ruled out.9
In 1938, Christie2
summarized the knowledge of this period by stating the following:
“although the conditions under which dyspnea occurs are diverse, giving the
impression of being complex, the main causes are few and relatively simple.
They consist of chemical and reflex disturbances. Chemical disturbances appear
to be the least important. Dyspnea is usually of reflex origin”. This was unÂdoubtedly
a reckless attempt to simplify the issue.
DYSPNEA AND BREATHING MECHANICS
At the beginning of this period,
the relationÂship between relative ventilation and ventilatory
capacity and its connection with dyspnea were recÂognized.8
If ventilation is expressed as a percentÂage
of the maximum ventilatory capacity (MVC),
ventilation should reflect the intensity of effort and of dyspnea. Decades
later, the ventilatory index (VE/MVC) would become a
synonym of dyspnea.10
In 1946, Rahn
and Otis were able to measure the impedance and forces involved in the act of
breathing in healthy subjects.10,
11 Their article has been significant in understanding the
breathing mechanics. Patients with heart failure or emphyÂsema had 2 to 4 times greater respiratory work than controls. In 1954,
Marshall et al suggested that dyspnea in patients with mitral stenosis and
emphysema was related to transpulmonary presÂsure
rather than respiratory work.12
In his classic experiment of
1954, Fowler demÂonstrated that the discomfort associated with voluntary apnea
could be relieved if the subject was allowed to take a few breaths from a bag
containing gases with the same composition as alveolar air. Surprisingly, even
though the levels of hypoxia and hypercapnia wouldn’t
change, the maneuver allowed the apnea to be maintained for an additional
period of time13 (Figure 1).
The implication of this study was that chemoreceptor activity (hypoxia and hypercapnia) didn’t seem to be the direct source of the
sensation that compelled the end of the apnea. Other observations in line with
this idea:
1. Hypercapnia
produced by adding CO2 to
inspired air causes less dyspnea if the respiratory pattern consists of large
thoracic movements.
2. Conversely, dyspnea increases
if thoracic moveÂments are voluntarily restricted below those corresponding to
a free pattern.
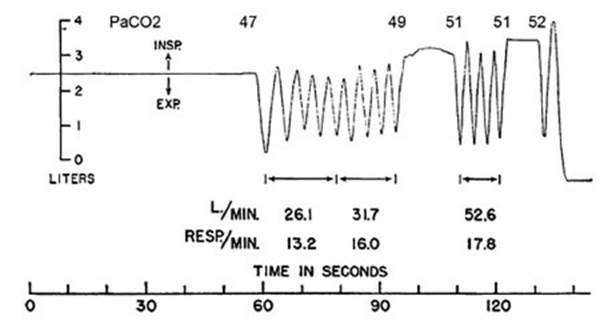
An interesting observation made
by Fowler13 in his study
was that “towards the end of voluntary apnea, strong involuntary contractions
of the respiratory muscles occur, and a significant volunÂtary effort is
required to prolong the apnea. The subjective relief occurs immediately upon
breaking the apnea, though less strongly after the second and third periods of
voluntary apnea”.
These observations led to the
understanding that the dissociation between the chemical drive to
breathe and the absence of thoracic movements during voluntary apnea
intensifies the sensation of dyspnea. During this period, it wasn’t possible to
reach a consensus on the most relevant mechaniÂcal factor causing dyspnea, but
the concept that breathing mechanics are important in the sensaÂtion of dyspnea
is now widely accepted.
DYSPNEA AND OXYGEN COST OF BREATHING
Due to the ease of obtaining
reliable and relatively quick measurements of oxygen consumption (VO2), the
focus shifted to understanding the relationship between dyspnea and the oxygen
cost of breathing (VO2resp).
VO2resp, which represents the oxygen
consumed by the respiratory muscles (and other movements associated with
breathing), serves as an index of the energy required for ventilation. Therefore, it was initially determined that VO2resp increases when ventilation and impedance
to the action of respiratory muscles increase.8, 14 In fact, since the diaphragm and most likely
other respiratory muscles obtain their energy almost totally through oxidative
metabolism across a wide range of respiÂratory work, changes in their energy
requirements can closely approximate the total VO2
.15
In 1958, Mc
Ilroy concluded this first stage by stating: “All conditions
in which dyspnea occurs, except respiratory paralysis,
share two common features: 1) a reduction in maximum oxygen upÂtake; and/or 2)
an increase in VO2resp […]. VO2resp can increase
due to abnormal lung or chest comÂpliance or resistance,
or due to abnormally high ventilation during exercise […]. Dyspnea may result
from inadequate supply of oxygenated blood to the respiratory muscles”.16
During this stage, the
relationship between dyspnea and VO2resp,
was developed, but it wasn’t possible to conduct an isolated analysis of these
variables.17 Recognizing
this fact led to the curÂrent concept that the analysis should consider
measurements of events related to metabolism, circulation, and breathing, along
with associated sensory events.18 This new approach
was shaped by the works of O’Donnell, Mahler, Killian, Jones, and others.2, 18-20
DYSPNEA AND INADEQUACY BETWEEN TENSION AND LENGTH
The effort of respiratory muscles
and the magniÂtude of ventilation required for common tasks like walking and
climbing stairs give rise to sensations that are recognized as adequate. It’s
possible to accurately classify the magnitude of tidal volume (VT), flow rate,
respiratory pressure, added resisÂtance, or elasticity. However, one rarely
becomes conscious of breathing until changes in the interÂplay of effort,
tension, length, and speed lead to the conscious sensation of inadequacy.10
The foundations of this
hypothesis were presÂent since Fowler’s time, but it was Campbell and Howell
who suggested that “an imbalance in the relationship between tension and
displacement of respiratory muscles could be the central mechaÂnism for
developing dyspnea”.21 According to
this hypothesis, dyspnea occurs when there is imbalÂance between the planned
change in length and the achieved length. In other words, dyspnea
arises when the achieved displacement is less than the expected displacement.21-23
As will be seen later, this
theory has been refined since then in order to include the general concept of a
mismatch between outgoing motor signals (efÂferents)
to the respiratory muscles and incoming information (afferent). The conscious
recognition of inadequacy is omnipresent across all sensory systems.10 For this reason, there are certain clinical observations
that are worth highlighting:
1. The sensation of dyspnea in
normal subjects can be evoked by breathing through a narrow tube or attempting
deep breaths while someone apÂplies pressure to the abdomen. In other words, an
attempt is made to move the chest and lungs, but there is an obstruction
preventing the respiÂratory muscles from shortening to achieve the expected
displacement.
2. The concept of inadequacy
seems to hold true even in the absence of mechanical load. When the respiratory
centers in the central nervous system are stimulated, increasing the drive to
breathe, dyspnea appears to worsen when chest wall movement is reduced. This
suggests that the lack of correspondence between efferent signals originating
from the brain and afferent signals returning from the chest wall results in a
sensation of breathlessness.
3. The immediate relief of
dyspnea that allows for chest movements after breaking voluntary apÂnea,13 without improving the blood gas status, is
also consistent with the concept of inadequacy or dissociation.
Which are the receptors for this
inadequacy? Although by the end of the 19th century it was recognized that
Golgi tendon organs mediated the sensation of tension, while joint receptors did
so for displacement (position and movement), muscle spindles were not
acknowledged as mediators of the sensation of displacement until the 1960s.12 These are
abundant in the intercostal muscles, and their afferent projections form spinal
and supraspinal reflexes. The diaphragm contains
tendon organs that detect tension and exert inÂhibitory influences on central
respiratory activÂity.24 This finding
allowed for the refinement of the inadequacy theory by appealing to the gamma
system (intrafusal fibers).10
If the shortening program is not fulfilled (inadequacy), this
information reaches sensory areas and dysÂpnea appears.
The concept of mismatch between
tension and length, along with all its derivations, stood the test of time. It
also allowed for the explanation of dyspnea caused by momentary suppression of
breathing during speech and swallowing, as well as the discomfort caused by
inadequate patient-respirator interaction.25
Its foundation would be further
supported in the coming decades with the introduction of the concept of
“efferent copy” or “corollary discharge,” along with brain mapping through
functional neuroimaging.
DYSPNEA IN DIFFERENT DISEASES AND CONDITIONS
The mechanisms of dyspnea have
been extensively studied in chronic obstructive pulmonary disease (COPD).19, 21-23, 26, 28 Regarding the
limits of tolerÂance, patients with moderate to severe disease consistently
report that the intensity of respiratory discomfort is severe and that each
inhalation feels unrewarded (unsatisfied inspiration).2
Neurophysiological constructs invoking a demand-capacity
imbalance or a neuromechanical dissociaÂtion provide
a reasonable theoretical basis for this dominant qualitative descriptor of
effort-related dyspnea in COPD.
As the severity of COPD increases,
there is a progressive decrease in the resting inspiratory capacity (IC) as a
result of lung hyperinflation. During exercise, ventilation increases in
response to higher metabolic demand, but the baseline state of hyperinflation
limits the increase in tidal volume. Respiratory rate increases to attempt to
maintain the VE, but this results in a shorter
expiratory time and thus greater hyperinflation, further limiting the VE
(Figure 2).
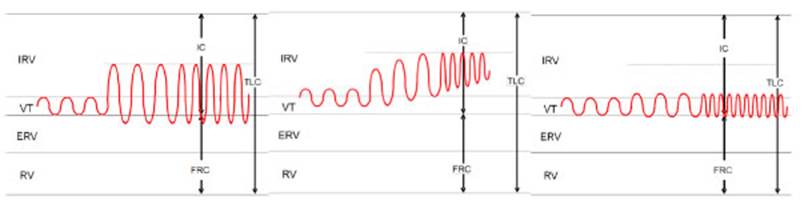
Dyspnea occurs as a consequence
of neuromeÂchanical uncoupling (high ventilatory drive with low effective ventilation) and the
stimulation of stretch receptors in the lung parenchyma and chest wall due to
hyperinflation. In patients with lower resting IC, the mechanical limit can be
reached earlier, and dyspnea becomes intolerable at the start of exercise. The
corollary is that even small increases in resting IC following an intervenÂtion
such as bronchodilator therapy or surgical/ endoscopic volume reduction
procedures delay the onset of critical neuromechanical
uncoupling and the resulting intolerable dyspnea.2, 28, 29
In response to increased
metabolic demand, ventilation increases through an increase in VT at the expense
of the inspiratory reserve volume (IRV) (to a greater extent) and expiratory
reserve volume (ERV). When VT reaches significant values (usually around 60% of
the forced vital capacity, FVC), further increases in ventilation occur through
an elevated respiratory rate (RR). In COPD patients, the basal IRV is reduced
due to air trapping, which limits the ability to increase VT. This is
compensated by a significant increase in RR, resulting in shorter expiratory
time and more air trapping.
In patients with thoracic
restrictive disease, a higher elasticity of the thoracic-pulmonary system
limits the increase in VT. Ventilatory increase is
primarily achieved through an elevated RR.
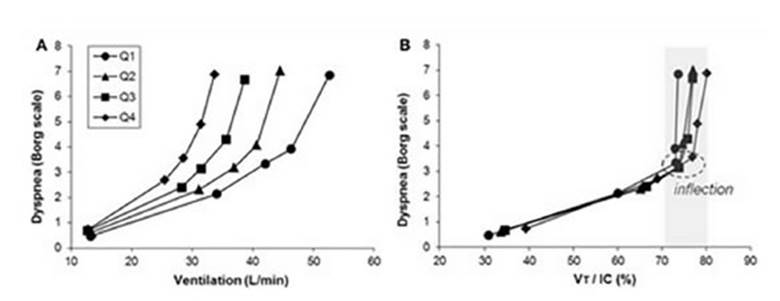
Panel A
of Figure 3 illustrates the relationÂship between dyspnea and incremental
exercise ventilation in COPD patients, grouped into four severity levels (Q1 to
Q4) based on FEV1 values.
It can be observed that similar ventilation levels produce more dyspnea as COPD
worsens (based on FEV1).
However, when the VT is normalized (panel B) according to the IC (VT/IC%), there is a turning point (70%-80%) common to all
severity levels, beyond which dyspnea notably increases.30
In bronchial asthma,
various factors influencÂing dyspnea have been identified, such as the rate of
airway obstruction development, use of medicaÂtion, psychological profile, and
the level of severity. But, differences in the intensity and quality of the
symptoms, both intra-individual and inter-indiÂvidual, can be largely
attributed to dynamic lung hyperinflation and its mechanical consequences,
including inspiratory threshold loading, reduced dynamic compliance and IC,
muscle weakness, and critical mechanical limitations in chest expansion, all
leading to the resulting neuromechanical disÂsociation
of the ventilatory system.
The mechanisms underlying dyspnea
in interÂstitial lung disease (ILD) are not fully underÂstood and have
not been extensively studied.29-31 However,
abnormalities in ventilatory mechanics, along with an
increased demand relative to capacÂity, contribute significantly to the
intensity and quality of dyspnea in these patients. As a result, interventions
that decrease ventilatory demand, improve ventilatory capacity, reduce mechanical load, or enhance
respiratory muscle capacity should alleviate dyspnea.24, 25 The concept of neuÂromechanical dissociation is highly relevant in these
conditions.
Despite their obvious
differences, pregnancy and obesity share ventilatory
and perceptual reÂsponses to the physiological stress of exercise.31-34 Over 70% of
pregnant women and obese adults (otherwise healthy) report dyspnea during daily
physical activities (for example, climbing stairs). Studies in obese
individuals and pregnant women support the following conclusions:
1. Mechanical/muscular
respiratory factors are not a major source of activity-related dyspnea during
pregnancy.
2. Gestational dyspnea reflects
awareness of increased VE and respiratory muscle effort acÂcompanying the rise
in neural motor drive (deÂtected by increased central corollary discharge to
sensory areas of the brain).
3. However, the increase in
dyspnea during physiÂcal activity in pregnant women cannot be easily explained
by mechanical/muscular respiratory factors or an increase in the central chemoreÂflex response, and presumably also peripheral
response.
The higher perception of
activity-related dysÂpnea experienced by many pregnant and obese individuals
likely reflects the awareness of an increase in the neural respiratory motor
drive needed to tolerate the greater ventilatory
demands of exercise in these specific populations.32, 33
CONCLUSIONS
All the theories had defenders
and detractors and, interestingly, with the development of sophisticatÂed
neurophysiological techniques and functional imaging it has been possible to
prioritize each of the mechanisms. All have survived the passage of time and
none can singularly explain dyspnea in all clinical situations, showing the
complex and multifactorial nature of the phenomenon.
It isn’t possible to overlook the
fact that patients with COPD and ILD, in addition to experiencing dyspnea
during exercise or at rest, often suffer from other symptoms such as general
fatigue, weight loss, depression, anxiety, loss of appetite, nausea, dry mouth,
and insomnia.34 Therefore,
these patients require a broader approach to their general sympÂtoms that can
further deteriorate their quality of life.
KEY POINTS
1. Unlike pain, the physiopathological mechanisms explaining dyspnea are complex
and can coexist. Depending on the clinical condition, one mechanism can be more
relevant than others. However, there are common denominators.
2. The explanation involving
neural reflexes was developed to account for the presence of dyspnea in the absence
of gasometric alterations, but it wasn’t sufficiently
convincing, so the role of altered breathing mechanics and the oxygen cost of
breathing were suggested.
3. Based on our current
knowledge, dyspnea occurs when there is imbalance between the demand for
breathing (central neural drive) and the capacity to breathe (respiÂratory
muscle function): tension-length inadequacy. This explanation gained
acceptance, in part because it integrated other mechanisms and was strengthened
by additional neurophysiological and imaging techniques.
Conflict of interest
Authors have no conflicts of
interest to declare.
REFERENCES
1. Mahler DA. Understanding
mechanisms and documentÂing plausibility of palliative interventions for
dyspnea. Curr Opin Support Palliat
Care. 2011;5:71-6.
https://doi.org/10.1097/SPC.0b013e328345bc84
2. Mahler DA, O’Donnell DE.
Dyspnea: Mechanisms, MeaÂsurement, and Management, Third Edition. CRC Press. 2014:256.
3. Meakins
J. A British Medical Association Lecture on the cause and treatment of dyspnoea in cardio-vascular disease. Br Med J. 1923;1:1043-5. https://doi.org/10.1136/bmj.1.3260.1043
4. Moosavi
SH, Golestanian E, Binks
AP, Lansing RW, Brown R, Banzett RB. Hypoxic and hypercapnic drives to breathe generate equivalent levels of
air hunger in humans. J Appl Physiol
(1985). 2003;94:141-54.
https://doi.org/10.1152/japplphysiol.00594.2002
5. Moosavi
SH, Banzett RB, Butler JP. Time course of air hunger
mirrors the biphasic ventilatory response to hyÂpoxia.
J Appl Physiol (1985). 2004;97:2098-103.
https://doi.org/10.1152/japplphysiol.00056.2004
6. Cullen GE, Harrison TR,
Calhoun JA, Wilkins WE, Tims MM. Studies in
congestive heart failure: XIII. The Relation of Dyspnea of
Exertion to the Oxygen Saturation and Acid- Base Condition of the Blood.
J Clin Invest. 1931;10:807-31.
https://doi.org/10.1172/JCI100385
7. Harrison TR, Calhoun JA,
Cullen GE, Wilkins WE, Pilcher C. Studies in
congestive heart failure: XV. Reflex Versus Chemical
Factors in the Production of Rapid Breathing. J Clin
Invest. 1932;11:133-54.
https://doi.org/10.1172/JCI100397
8. Mahler D. Dyspnea. Medicine
& Science in Sports & ExerÂcise 1991;23:1322.
9. Gesell R, Moyer C. Is
breathing fundamentally a reflex pheÂnomenon? [Internet].
Quarterly Journal of Experimental Physiology 1935; 25:13–31.
10. Donald A. Mahler DO. Dyspnea, Mechanisms, Measurement and
Management. Donald A. Mahler DO, editor. CRC Press;
2005. (2nd Edition).
11. Rahn H, Otis AB, Chadwick LE,
et al. The pressure-volume diagram of the thorax and lung [Internet].
American Journal of Physiology-Legacy Content. 1946; 146: 161-78.
https://doi.org/10.1152/ajplegacy.1946.146.2.161
12. Marshall R, Stone RW,
Christie RV. The relationship of dyspnoea
to respiratory effort in normal subjects, mitral stenosis and emphysema.
Clin Sci. 1954;13:625-31.
13. Fowler WS. Breaking
Point of Breath-Holding [Internet]. J Appl Physiol 1954; 6: 539-45.
https://doi.org/10.1152/jappl.1954.6.9.539
14. Cournand A, Richards DW Jr, Bader RA, Bader ME, FishÂman AP. The oxygen cost of
breathing. Trans Assoc Am Physicians. 1954;67:162-73.
15. Zakynthinos
S, Roussos C. Oxygen Cost of Breathing. In: Gutierrez G, Vincent JL. (eds) Tissue
Oxygen UtilizaÂtion. Update in Intensive Care and Emergency MediÂcine,
1991;12. Springer, Berlin, Heidelberg.
https://doi.org/10.1007/978-3-642-84169-9_14.
16. McIlroy
MB. Dyspnea and the work of breathing in diseases of the
heart and lungs [Internet]. Progress in CardiovasÂcular
Diseases 1958; 1: 284-97. https://doi.org/10.1016/S0033-0620(59)80027-X
17. Harden KA, Bartlett RG,
Barnes H, Reid L, Barthakur A, Waters WP. Oxygen cost
of breathing. I. Am Rev Respir Dis. 1962;85:387-91.
18. Killian KJ, Jones NL. Mechanisms of exertional dyspnea.
In: Killian KJ, Jones NL. Mechanisms of exertional
dyspnea. Clin Chest Med., editor. Clinical
exercise TestÂing. Philadelphia, Saunders; 1994.
247-57. https://doi.org/10.1016/S0272-5231(21)01071-6
19. American Physiological
Society. Exercise: Regulation and Integration of Multiple Systems. Am
Physiological Society 1996; 1210 p.
20. Campbell EJ, Howell JB. The sensation of breathlessness. Br Med Bull. 1963 Jan;19:36-40.
https://doi.org/10.1093/oxfordjournals.bmb.a070002
21. Bennett ED, Jayson MI, Rubensteind, Campbell EJ. The ability of
man to detect added non-elastic loads to breathÂing. Clin
Sci. 1962;23:155-62.
22. Campbell EJ, Freedman S,
Smith PS, Taylor ME. The abilÂity of man to detect added
elastic loads to breathing. Clin Sci. 1961;20:223-31.
23. Dyspnea. Mechanisms, assessment,
and management: a consensus statement. American Thoracic
Society. Am J Respir Crit
Care Med. 1999;159:321-40.
https://doi.org/10.1164/ajrccm.159.1.ats898
24. Manning, Harold L., Schwartzstein, Richard M. PathoÂphysiology of Dyspnea. New
England Journal of MediÂcine [Internet] 1995;333:1547
https://doi.org/10.1056/NEJM199512073332307
25. Anzueto
A, Miravitlles M. Pathophysiology of dyspnea in COPD.
Postgrad Med. 2017 Apr;129(3):366-74. https://doi.
org/10.1080/00325481.2017.1301190
26. Hanania
NA, O’Donnell DE. Activity-related dyspnea in chronic obstructive pulmonary
disease: physical and psychological consequences, unmet needs, and future direcÂtions.
Int J Chron Obstruct Pulmon Dis. 2019;14:1127-38.
https://doi.org/10.2147/COPD.S188141
27. O’Donnell DE, Laveneziana P, Webb K, Neder JA.
ChronÂic obstructive pulmonary disease: clinical integrative physiology. Clin Chest Med. 2014;35:51-69.
https://doi.org/10.1016/j.ccm.2013.09.008
28. Denis E. O’Donnell, Amany F, et al. Exertional dyspnoea in COPD: the clinical utility of cardiopulmonary
exerÂcise testing. Eur Respir
Rev 2016;25:333-47
https://doi.org/10.1183/16000617.0054-2016
29. Miki K, Maekura
R, Miki M, et al. Exertional acidotic reÂsponses in
idiopathic pulmonary fibrosis: The mechanisms of exertional
dyspnea [Internet]. Respiratory Physiology & Neurobiology 2013;185:653-8. https://doi.org/10.1016/j.resp.2012.11.008
30. O’Donnell DE, Elbehairy AF, Berton DC, Domnik NJ, Neder JA. Advances in the Evaluation of Respiratory Pathophysiology during
Exercise in Chronic Lung DisÂeases. Front Physiol. 2017;8:82. https://doi.org/10.3389/fphys.2017.00082
31. O’Donnell DE, Ora J, Webb KA, Laveneziana P,
Jensen D. Mechanisms of activity-related dyspnea in pulmonary disÂeases. Respir Physiol Neurobiol. 2009;167:116-32.
https://doi.org/10.1016/j.resp.2009.01.010
32. Pham K, Schaeffer M, Reid R, Abdallah S, Andersen R, Jensen D. Physiological mechanisms
of increased activity-related dyspnea in obesity [Internet]. 4.1 Clinical
respiraÂtory physiology, exercise and functional imaging. 2015.
https://doi.org/10.1183/13993003.congress-2015.PA2234
33. Jensen D, Webb KA, Wolfe LA,
O’Donnell DE. Effects of human pregnancy and advancing gestation on respiraÂtory
discomfort during exercise [Internet]. Respiratory Physiology &
Neurobiology 2007, 156: 85–93. https://doi.org/10.1016/j.resp.2006.08.004
34. Rantala
HA, Leivo-Korpela S, Lehto
JT, Lehtimäki L. Dyspnea on Exercise Is Associated
with Overall Symptom Burden in Patients with Chronic Respiratory Insufficiency.
Palliat Med
Rep. 2021;2:48-53.
https://doi.org/10.1089/pmr.2020.0112