Autor :Saraguro RamĂrez, Byron Leonel1 , Jaramillo, Byron1, Zuna, Efrain1 , GarcĂa, Artemio1
1 Pulmonologist, Hospital General Instituto Ecuatoriano de Seguridad Social; Babahoyo, Ecuador Advanced Course on Respiratory Endoscopy AABE (Argentine Association of Bronchoesophagology)
https://doi.org/10.56538/ramr.XBEE1703
Correspondencia : Byron Leonel Saraguro RamĂrez. E-mail: byronsaraguromd@gmail.com
ABSTRACT
Introduction: The adenoid cystic carcinoma of the airway is a rare tumor that
originates from the submucosal glands of the
tracheobronchial tree. Due to the usual delay that occurs between symptoms and
diagnosis, and the propensity of this tumor to expand through the perineural pathways and submucosa,
the recommended treatment is surgical resection with postoperative radiation
therapy. Survival is determined by the presence of distant metastasis.
Case report: 70 year-old female patient with a history of arterial hypertension, COPD
(former smoker, 34 pack/years) who came to the Emergency Service with episodes
of hemoptysis and previous dyspnea with a score of 3-4 according to the mMRC (modified Medical Research Council) dyspnea scale.
Discussion: Malignant neoplasms of the trachea are very rare, and data related to
them is limited. The most important prognostic factors in primary malignant tumors
of the trachea are: early diagnosis, cancer staging, histology, and treatment
options.
Conclusions: Early detection may be associated with increased resectability
rates and even prolonged survival.
Key words: Hemoptysis, Adenoid cystic carcinoma, Bronchoscopy, Surgery, Radiation
therapy
RESUMEN
IntroducciĂłn:
El
carcinoma adenoide quĂstico de la vĂa aĂ©rea es un
tumor poco comĂşn, que se origina de las glándulas submucosas del árbol traqueobronquial. Por el usual retraso entre los sĂntomas y
el diagnóstico, y por la propensión de este tumor para expandirse a través de
los haces perineurales y submucosa, el tratamiento
recomenÂdado es la resecciĂłn quirĂşrgica con radioterapia posoperatoria. La
supervivencia está determinada por la presencia de metástasis a distancia.
Caso
clĂnico: Paciente
de sexo femenino de 70 años de edad con antecedentes de hipertensión arterial,
EPOC (extabaquista 34 paquetes/año) que acude a
servicio de urgencias con episodios de hemoptisis y disnea mMRC
3-4 previa.
DiscusiĂłn:
Las
neoplasias malignas de la tráquea son muy raras y los datos relaÂcionados con
ellos son limitados. Los más importantes factores pronósticos en las
enfermedades primarias malignas de la tráquea constituyen el diagnóstico
temprano, estadiaje del tumor, histologĂa y opciones
de tratamiento.
Conclusiones:
La
detecciĂłn temprana puede estar asociada con el incremento de las tasas de resecabilidad e, incluso, supervivencia prolongada.
Palabras
clave: Hemoptisis,
Carcinoma adenoide quĂstico, Broncoscopia,
CirugĂa, Radioterapia
Received: 11/08/2022
Accepted: 03/28/2023
INTRODUCTION
Primary tracheal tumors account
for less than 1%1 (0.1% to
0.4%) of all malignant respiratory diseases.2
They are typically malignant in adults
(80-90%) and benign in children (60-70%).3
A primary tracheal carcinoma is a
malignant tumor located between the first tracheal ring and the carina.4 Data related
to these tumors are limÂited due to the small number of cases reported in
publications, posing a diagnostic and therapeutic challenge.5
Squamous cell carcinoma comprises
two-thirds of primary tracheal tumors in adults. It usually presents around the
age of 60 and predominantly affects men. Adenoid cystic carcinoma is the secÂond
most common malignant tracheal tumor after squamous cell carcinoma.6 It is
typically found in young patients, occurring between the fourth and fifth
decades of life, and affects both men and women equally.7
Adenoid cystic carcinoma, also
known as cylinÂdroma, was first reported by Billroth in 1856. It is a rare malignant tumor of the head
and neck, accounting for approximately 10% of tumors loÂcalized in this region.
It originates in the salivary glands, most commonly in the parotid gland.8 RareÂly, it can occur in the trachea9
originating in the submucosal glands of
the tracheobronchial tree. Depending on its location, it can be classified as
laÂryngeal (including subglottis) or
tracheobronchial. Laryngeal adenoid cystic carcinoma is extremely rare, with
approximately 40 cases reported in the last 41 years. Most adenoid cystic
carcinomas deÂvelop centrally in the trachea (64.6%) and main bronchi (19.5%).
The primary presenting symptom is
often dysÂpnea. Definitive diagnosis is delayed, and most cases are diagnosed
when the disease is already at an advanced stage.
Recent studies, such as the one
by Hämetoja et al, have shown that the JC polyomavirus (JCPyV) can be found
in samples from minor salivary glands and detected through quantitaÂtive
polymerase chain reaction (qPCR). However, the
prevalence of JCPyV positivity in adenoid cystic
carcinoma was low, and the viral copies detected were insufficient to establish
its role in the carcinogenesis of this tumor.10 Other studies
suggest a potential role of the human papillomavirus (HPV) in the
carcinogenesis of adenoid cystic carcinoma.
Selection criteria and treatment
indications for this condition are not consistent. Even patients with resectable disease are often managed with palliative treatment,
likely due to the lack of available prospective studies that evaluate and
compare treatments, which are almost impossible to conduct given the rarity of
these tumors.
The choice of treatment modality
clearly affects survival. Surgery has been shown to be superior to radiation
therapy in many studies. The 5-year survival rate varies between 41% and 57%
with surgical management. In patients treated with raÂdiation, the 5-year
survival rate varies from 6% to 11%. For this reason, surgery should be considered
in most cases, including those of advanced disease.
The delicate arterial and
lymphatic network could explain the rarity of hematogenous
meÂtastases and the relative frequency of regional lymph node metastases at
initial presentation. Late metastases can occur more commonly in the lungs, but
this condition can also spread to the brain, bones, liver, thyroid, and spleen.
Adenoid cystic carcinoma is also known for its tendency to cause neutropenia
and for recurring locally or regionally many years after initial presentation
and treatment. The relatively low incidence of adenoid cystic carcinoma in the
periphery of the lung is likely associated with the distribution of glandular
cells. In a review of 15 cases, Moukarbel et al
reported a local recurrence rate of 33% and a distant metastasis rate of 67%,
primarily to the lungs.
Epidemiology
In Argentina, in the year 2017,
cancer-related mortality was reported at 118 and 87 deaths per every hundred
thousand males and females, reÂspectively. Lung cancer determined the highest
number of deaths among malignant tumors in 2017, with 9,485 deaths, accounting
for 15% of all cancer-related deaths and 20% of deaths from this cause in
males.11
In the United States, adenoid
cystic carcinoma represents 2 cases per one million people annually. Other data
report 2 to 6 new cases per million people each year, accounting for less than
0.1% of cancer deaths per year.12
Due to the rarity of the adenoid
cystic carciÂnoma of the airway, prospective studies that assess prognostic
factors, treatment, and outcomes are not feasible and do not allow for external
validity determination. Therefore, cases from institutional series serve as an
important guide for the theraÂpeutic approach.
Cigarette smoking history is
commonly associÂated with squamous cell carcinoma of the trachea; however,
there are no specific risk factors associÂated with adenoid cystic carcinoma.
Pathology
Primary tracheal tumors can originate
from the respiratory epithelium (squamous cell carcinoma, adenocarcinoma), the
salivary glands (adenoid cystic carcinoma, mucoepidermoid
carcinoma), and mesenchymal structures (sarcoma,
lymphoma).
Adenoid cystic carcinoma is a
distinct type of carcinoma that arises from both major and minor salivary
glands. Less commonly, it can originate in the seromucinous
glands of the upper and lower respiratory tract, which have been shown to
decrease from the supraglottis to the glottis, subglottis, and trachea.13
According to the World Health
Organization classification, adenoid cystic carcinoma is defined as a basaloid tumor composed of epithelial and myoepithelial cells with various morphological
configurations, including tubular, cribriform, and solid patterns.14 The cribriform pattern is the most common, characterized by
uniform cells arranged in nests separated by cystic spaces containing mucinous
material.
Histologically, these tumors
consist of two main cell types: ductal (luminal) and myoepithelial
(abÂluminal) cells.
Macroscopically, adenoid cystic
carcinoma exhibits exophytic nodular growth, leading
to stenosis of the tracheal lumen. It has a propensity to spread along the submucosal and perineural planes,
with only 10% of patients developing lymph node or distant metastases. The
presence of perineural invasion is a distinctive
feature of the tumor and is associated with a tendency to develop recurrent
disease after surgical resecÂtion, likely due to the higher likelihood of microÂscopic
residual disease at the resection margins or beyond and its strong propensity
to invade the nerves.15
The pathological distribution is
related to the prognosis. Adenoid cystic carcinoma has shown greater survival,
compared to squamous cell carÂcinoma
Mitsuaki et al described an abrupt transformation of a low-grade or
well-differentiated tumor within a tumor with a high-grade component without a
specÂtrum of the original tumor; this is called undifferentiÂated adenoid
cystic carcinoma of the trachea, which is an extremely rare and highly
aggressive tumor.16
Clinical presentation
Tracheal tumors can often go
undiagnosed for months or even years due to their slow growth and silent
nature, and are typically discovered at a later stage. In a study including 52
patients between March 1995 and March 2012, Chen et al described that the
average duration of symptoms before diagnosis was 18 months, with a range from
1 to 98 months.
Many patients present with
symptoms such as dyspnea, wheezing, and chronic cough, which are often confused
with conditions like asthma. In smokers, these symptoms can be mistaken for
chronic obstructive pulmonary disease or chronic bronchitis, because these
tumors do not cause symptoms until they occlude more than 50% of the tracheal
lumen diameter. Exertional dyspnea does not typically
develop until the trachea has reduced its lumen to less than 8 mm, and once the
lumen becomes less than 5 mm or 75% of its original size, dyspnea can also
occur at rest.17 This tumor
can progress to the point of compromising the airway and causing fatal
secondary asphyxia, as reported by Huston et al.
In a study involving 82 patients
at the ShangÂhai Chest Hospital in China from March 2001 to April 2012, Zhao et
al described that the primary symptom of tracheal adenoid carcinoma was dysÂpnea
(66%), followed by cough (13.2%), hemoptysis (13.2%), and stridor (3.8%).
Irritation or ulceration of the
mucosa can lead to cough and hemoptysis, while the invasion of adjacent
structures can result in dysphagia. Distant metastases occur in less than 10%
of patients.
Hemoptysis is the main symptom in
patients with squamous cell carcinoma, and typically leads to early diagnosis
within 4 to 6 months. Adenoid cystic carcinoma presents with wheezing or striÂdor
as the primary symptom, and less than 25% of patients experience early
hemoptysis in its course, which explains why symptoms can last for
approximately 18 months before a definitive diagnosis is made.
Diagnosis
Patients with symptoms such as
shortness of breath and wheezing that do not respond to bronÂchodilator
treatment should prompt us to consider a tracheal tumor among the differential
diagnoses.
Lung function tests, for example
the spirometry, can reveal fixed upper airway
obstruction, evidencÂing impairment in both inspiratory and expiratory
flow-volume curves.
Chest X-rays are rarely
diagnostic. The most useful method for assessing the extent and relaÂtionship
of the tumor with adjacent structures is a CT scan. Imaging studies with multiplanar and three-dimensional reconstruction with
internal (virtual bronchoscopy) and external views can demonstrate whether the
lesion is inside the luÂmen, outside the airway, or has characteristics of
both.18 The presence
of a soft tissue mass in the trachea with increased uptake of 18-fluoroÂdeoxyglucose
(18F-FDG)
on positron emission toÂmography with multi slice computed tomography
(PET/MSCT) is highly suggestive of a malignant tracheal tumor.
The bronchoscopy is a valuable
tool for the diagnosis and staging of tracheal tumors because it allows for the
collection of tissue samples and the evaluation of the location and extent of
the disease, as well as the relationship between the length of the tumor and
the trachea. The endoÂscopic ultrasound can also determine the degree of
tracheal invasion.13
Bronchoscopic findings may reveal a large mass or circumferential lesion within the
trachea. The appearance of the tumor can vary, but it is preÂdominantly red,
granular, or fleshy, and easily friable. The borders of the lesion may be
poorly defined or diffusely infiltrative. The margins of protruding masses may
also show mucosal elevaÂtion or vascularity, providing evidence of infiltraÂtion
beneath the mucosa.
Treatment
Malignant primary tumors are
usually treated with surgery, endoscopic resection through variÂous techniques,
and radiation therapy. However, only surgery can cure benign tumors and
low-grade malignant tumors, achieving long-term survival in tracheal carcinomas.
Surgery also provides complete pathological confirmation of the tumor and
permanently relieves airway obstruction.
Identifying the extent of local
disease is the most important factor in determining the therapeutic management.19 The decision to resect or
irradiate the tracheal tumor will depend on many factors, including the
patient’s overall health, tumor hisÂtology and location, and the length of the
airway that could be preserved after resection.
If a patient has life-threatening
airway obstrucÂtion, resection with rigid bronchoscopy may be used to delay the
surgery. However, management with stents or non-adjuvant radiation therapy is
not recommended unless the resection cannot be performed.
Surgery
Surgery is the cornerstone of
treatment for adÂenoid cystic carcinoma, and requires a high level of
expertise. It is applicable to patients with localÂized disease and has been
associated with a better long-term prognosis.20
Compared to other head and neck
cancers, adenoid cystic carcinoma is more challenging in terms of surgical
resolution, often resulting in positive margins.
Complete resection is achieved in
42%-57% of cases. It is associated with better survival and is essential due to
the high recurrence rate when a residual tumor remains. There is a higher risk
of local recurrence and positive surgical margins when the tumor is located in
the distal trachea.
Some of the surgical techniques
for treating tracheal tumors are: laryngectomy with
resection of the upper trachea, larynx, and trachea; tracheal resection; carinal resection without lung resecÂtion; and carinal resection with lung resection. Laryngotracheal
resection should be preferred over laryngectomy for
subglottic tumors. Jiao et al described a new minimally invasive surgical
technique in which they performed circumferential tracheal resection and
end-to-end anastomosis via thoracoscopy, taking into
consideration facÂtors such as tumor size, location, local invasion of the
lesion, and the surgeon’s experience. This approach was found to be safe,
effective, and could serve as a new alternative strategy for treating distal
tracheal tumors.21
In cases of bronchial
localization, some of the surgical techniques include pneumonectomy,
carinal resection without lung removal, carinal resection with lung
removal, sleeve lobectomy, and lobectomy. The use of deltopectoral
flaps with costal cartilages has been found to be satÂisfactory.
Absolute contraindications for
surgery include the presence of numerous positive lymph nodes, involvement of
more than 50% of the trachea, mediastinal invasion of
unresectable organs, mediastinum that has received a
maximum radiation dose of more than 60 Gy, or
previous surgery for distant metastases of squamous cell carcinoma.
For a minority of patients (less
than 20%) who present with metastatic disease, resection may be purely
palliative, aimed at relieving airway obstruction in cases where a tracheostomy
is not feasible.
Bronchoscopy
It is a useful technique for the evaluation
and, in several cases, the palliative treatment of the respiratory airways by
reducing the tumor or in unresectable patients for
stent placement.
Endotracheal tumors can be
resected endoÂscopically for palliation in inoperable
patients (e.g., patients with stage T4N3 or higher) or as a means to keep the
airway permeable until definitive resecÂtion can be performed. Tumors can be
removed usÂing biopsy forceps and suction, electrocoagulation, cryotherapy, laser therapy, photodynamic therapy, or argon
plasma coagulation. These measures should never be used as curative attempts
because they rarely offer long-term survival.
Sato et al described the
multi-session endoscopic treatment with argon plasma coagulation in a patient
with tracheal adenoid cystic carcinoma. They demonstrated its safety by
producing less vapor and smoke, controlling the depth of coagulation (up to 3
to 4 mm maximum), and ensuring safe and effective coagulation, especially in
large areas. This method was considered a safe palliative therapy, similar to
other methods like electrocauÂtery or Nd-YAG laser for tumor control, with fewer adverse
reactions.22
Endobronchial stents
In patients with unresectable or medically inopÂerable lesions, reliable and
durable palliation can be achieved in 80%-90% of appropriately selected
patients through the use of expandable or silicone stents.
Both silicone and self-expanding
metallic stents (SEMS) are widely used in case of airway stenosis.
One type of SEMS, known as the
AERO stent, combines the characteristics of a metallic and silicone stent
covered with a nitinol structure. Its advantages
include being insertable via flexÂible bronchoscopy,
easy removal, strong expanÂsion properties, and a lower risk of migration.
However, it is associated with a higher risk of infection compared to other
stents. Nonetheless, it provides an effective means to improve the paÂtients’
quality of life.23 Himeji et al
described two cases of tracheal stenosis secondary to malignant disease in
which they used a self-expanding meÂtallic stent (SEMS). They reported no
identified complications and improvement in obstructive symptoms, resulting in
a clear enhancement of the patients’ quality of life.
Radiation therapy
Radiation therapy is indicated as
definitive therapy for primary unresectable lesions,
in medically inoperable patients, as adjuvant treatÂment after resection, and
for palliation of severe symptoms. Radiation therapy alone has typically been
reserved for advanced or unresectable cases.
Postoperative radiation therapy should be used in most patients, but
post-surgical radiation is also a treatment of choice because surgical margins
are often involved.
Negative margins and adjuvant
postoperative radiation therapy are associated with improved survival prognosis.
Incomplete resection can be
converted to comÂplete resection by administering 60 Gy
of postÂoperative photon radiation therapy, given as five fractions of 2 Gy per week for more than 6 weeks. This treatment
eliminates microscopic residual carcinoma in the tumor bed and regional lymph
nodes. For macroscopic residual carcinoma, the required doses should be
increased to 68-70 Gy, administered as 5 fractions of
2 Gy for more than 7 weeks.
High-dose endobronchial
therapy with IridiÂum-192 has been reported to yield good palliative results
with minimal toxicity. However, a small study involving four tracheal neoplasms
treated with endobronchial Iridium-192 reported
tracheal stenosis in two long-term survivors.
Endotracheal brachytherapy could
be a reasonÂable approach for tracheal carcinomas, as it has shown to improve
local tumor control when used after 60-68 Gy of
external beam radiation therapy at doses of 8-15 Gy.
It is commonly used for traÂcheobronchial obstruction but can potentially lead
to life-threatening bleeding and airway erosion, requiring surgical
intervention.
Chen et al reported that
postoperative radiation therapy was only used for patients with positive
margins and observed a significant improvement in overall survival and
disease-free survival in these patients compared to those who received only
incomplete resection without radiation therapy.
A study conducted by Bittner et
al between 1989 and 2005, reported 20 patients with adenoid cystic carcinoma
treated with fast neutron radiotherapy at the University of Washington. They
considered it to be an effective treatment for locally advanced adenoid cystic
carcinoma that may offer therapeuÂtic benefits over commonly used treatment
modaliÂties. They reported a 5-year overall survival rate of 89.4% and a 5-year
local control rate of 54.1%, primarily in patients with unresectable
disease or locally advanced disease.24
Neutron radiation therapy has proven to be effective in advanced
or unresectable carcinoma and has been used in
single-institution experiences with low morbidity rates, although some cases of
tracheal cartilage stenosis or necrosis have been described.
In their case series, Levy et al
described acute toxicity related to moderate-grade radiation therapy in all
their patients. They reported esophagitis in 42% of cases, dysphonia in 32%,
and mucositis in 9%. One patient experienced tracheoesophageal fistula during treatment. With regard to
late toxicity signs, 7 patients (23%) developed symptomatic tracheal stenosis,
and 5 (12%) required subsequent tracheotomy. Grade 3 dyspnea occurred in 4
patients (14%); and 5 patients (16%) developed hypothyroidism. 4 paÂtients
(12%) showed pericarditis. In the study of Chen et al, the most common adverse
reactions were tracheitis and esophagitis.
Major complications following
conventional radiotherapy, such as chondronecrosis,
usually occur between 3 and 12 months after treatment.
Doggett et al performed
percutaneous implanÂtation of radioisotope seeds guided by computed tomography
in three patients diagnosed with adenoid cystic carcinoma. Two of them had
previÂously undergone tracheal resection, laser ablation, and post-operative
radiotherapy, and one patient declined resection and radiation therapy. All
three patients responded well in the short term and during nine months of
follow-up without chronic adverse effects and with reduced or relieved coughÂing.
However, long-term follow-up is necessary to assess efficacy and toxicity.12
Chemotherapy
Few studies have shown the role of
chemotherapy in the treatment of tracheal adenoid cystic carciÂnoma, and
further studies are needed to clarify its efficacy. Chemotherapy does not have
a significant role as primary therapy, but it may be considered for palliative
treatment in cases of distant metaÂstatic disease, for radiosensitization,
or in comÂbination with radiation therapy for unresectable
carcinomas.
Cisplatin-based chemotherapy has been used successfully in a patient with an unresectable tumor in combination with radiation. However,
this form of treatment has not been prospectively evaluated yet in primary
tracheal tumors.
Tracheal adenoid cystic
carcinomas are generÂally considered chemoresistant.
Chemotherapy and targeted therapies administered alone are not indicated for
localized tumors. Responses to cheÂmotherapy regimens based on cisplatin, cyclophosÂphamide, and adriamycin have been evaluated.
Alternative treatments such as
brachytherapy, photodynamic therapy, and cryotherapy
are availÂable but have not shown significant long-term benefits and are
primarily used for palliation.
CASE REPORT
We present the case of a
70-year-old female patient with a history of arterial hypertension, former
smoker (34 pack-years), diagnosed with COPD who presented with a hemoptysis
episode. She had previously experienced dyspnea classified as functional class
III-IV, which was managed as an outpatient exacerbation of COPD. A chest CT
scan was requested, revealing a soft tissue density lesion located in the
distal third of the trachea (Fig. 1 and 2), causing a narrowing of its lumen.
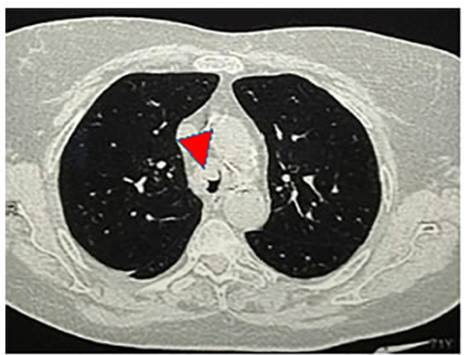
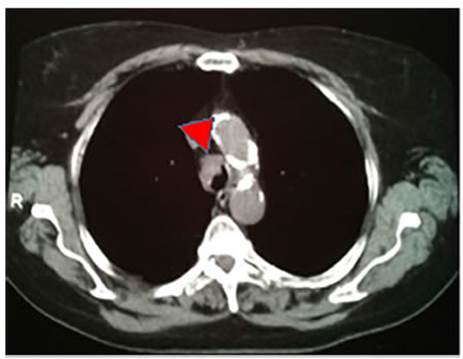
Tumor resection was performed
through rigid bronchoscopy, revealing 80% obstruction of the tracheal lumen in
the distal third area, associated with malacia and
mucosal infiltration extending to both main bronchi. Two lesions were removed,
one of 4 Ă— 1.2 cm and another one of 1.4 Ă— 1.2 cm (Fig. 3). The histopathological report of the lesions showed tracheal
mucosa infiltrated by an atypical proliferation consisting of cribriform,
tubular, and solid nests, lined by a biphasic population of inner cells with eosinophilic cytoplasm, round nuclei, and granular
chromatin. There was an outer layer of cells with clear cytoplasm, round and
oval nuclei with occasional isolated nucleoli, and basophilic intraluminal mucoid material. Positive AE1/AE3, negative TTF1 (thyroid
transcription factor 1), S100 nuclear and cytoplasmic staining in myoepiÂthelial cells, positive AML (acute myeloid leukeÂmia)
in myoepithelial cells, positive CALPONIN in myoepithelial cells, and positive CK7 in the inner layer,
consistent with adenoid cystic carcinoma with resection margins compromised by
the lesion.
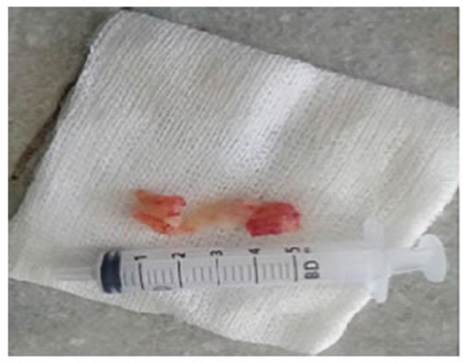
The case was presented in a
medical conference with the oncology department, where surgical resection was
ruled out due to mucosal extension of the lesion to the carina and both main
bronchi. A tracheobronchial Y-stent was placed, and the patient was clinically
and endoscopically followed-up, on a regular basis.
DISCUSSION
Adenoid cystic carcinoma is a
malignant tumor of the salivary glands that is relatively common in the head
and neck region. However, its presence in the airway is rare.
The mean age of presentation is
under 50 years, but the patient of this case was diagnosed at the age of 70,
thus falling within the age range reported by Zhao et al, Calzada
et al, Webb et al, and Chen et al. However, it correlates with the commonly
described late diagnosis.
There is no gender predilection
for the presentaÂtion of adenoid cystic carcinoma; however, Webb et al, Chen et
al, and Levy et al reported more cases in female patients.
No specific risk factors have
been associated with the presentation of adenoid cystic carcinoma, as reported
by Webb et al. The patient was a forÂmer smoker with a smoking cessation period
of 35 years. Calzada et al found in their patient
series that 36% of the cases were smokers.
The mean duration of symptoms
before diagÂnosis is considered to be 18 months, with a range between 1 to 98
months. Dyspnea is the most common initial symptom, as reported by Zhao et al
and Webb et al, so it can be underdiagnosed and confused with conditions such
as asthma, COPD, or chronic bronchitis, thereby delaying the definiÂtive
diagnosis. The patient initially presented with functional class III-IV
dyspnea, which, due to her clinical history, was initially considered as COPD
exacerbation. Bronchoscopy was initially chosen as the therapeutic approach due
to hemoptysis, since it is the second most frequent symptom following dyspnea,
as reported by Webb et al.
Regarding the location, Webb et
al, Chen et al, and Zhao et al described in their case series a predominance of
tumors in the lower third of the trachea, similar to the patient described in
this case. The macroscopic size of the tumors ranged from 1.5 cm to 8 cm, with
an average of 3.1 cm, correlating with the macroscopic size of the tumor of our
patient, which measured 4 x 1.2 cm; and had another one of 1.4 Ă— 1.2 cm.
Tracheal adenoid cystic carcinoma
is associÂated with very poor local and regional control, as demonstrated in
40% of patients in some series. Obtaining negative surgical margins is more
challenging due to the relative inability to resect more than 6 cm of the
trachea and the poor outcomes associated with tracheal grafts. Calzada et al reported that 80% of patients with adenoid
cystic carcinoma had positive margins, predominantly in the distal trachea, and
40% had locoregional recurrences, which is consisÂtent
with the findings described by Zhao et al. The patient’s histopathological
report indicates that the resection margins are compromised by the lesion.
Calzada et al highlight in their study the tendenÂcy for local recurrences of
adenoid cystic carcinoma in cases with positive margins and a distal location
in the trachea, as is the case with the patient we describe. During the
follow-up period ranging from 4 to 168 months (average 31 months), two patients
experienced local recurrence of the disease. One of them had a recurrence at 2
months, and the second at 1 month after surgery. One of these patients died at
16 months post-surgery, being the youngest in the series at 25 years of age.
Surgery is the cornerstone of
treatment for adenoid cystic carcinoma; however, Ahn
et al used laser removal through bronchoscopy in 2 out of 18 patients, which
was successfully performed in selected cases of early-stage tumors. The patient
came to the Emergency Service with hemoptysis. Endoscopic assessment and
subsequent tumor reÂmoval through rigid bronchoscopy were performed.
In the review by Benissan-Messan et al, patients with adenoid cystic
carcinoma were four times more likely to undergo resection, and survival was
significantly higher for patients who underÂwent resection with curative
intent. The overall mortality within 90 days following surgery was 2.5%,
showing low perioperative mortality and a favorable long-term prognosis.
In their case series, Levy et al
stated with regard to prognostic factors that the absence of perineuÂral invasion and a dose of radiation therapy of ≥
60 Gy correlate with better outcomes. Webb et al
reported that 20 of 74 patients (27%) developed distant metastasis either as an
initial presentation or during the follow-up period. Results were better for
patients without lymph node metastasis or disÂtant metastasis. Garden et al
determined that the presence of perineural invasion
of small nerves is not associated with worse control. Positive margins and
involvement of major nerves were associated with an increased risk of local
failure in patients treated with surgery and radiation.
Regarding mortality, Ahn et al did not find a significant difference between
squamous cell carÂcinoma and adenoid cystic carcinoma but noted that pulmonary
metastases were the primary cause of death in adenoid cystic carcinoma (6 out
of 7 cases).
The 5-year mortality rate
reported by Webb et al was 72.9%. Patients with adenoid cystic carcinoma and
those with primary cervical tumors had better overall survival rates than other
patients.
Zhao et al reported that survival
after resection of all adenoid cystic carcinomas was 93.9% at 5 years and 61.1%
at 10 years. In contrast, disease-free survival was 73.9% at 5 years and 26.9%
at 10 years.
Early diagnosis, experienced
surgical treatÂments, and adjuvant postoperative radiation therÂapy for
selected patients with positive margins can contribute to improving the
survival of patients with primary tracheal adenoid cystic carcinoma.
The lack of a standardized
staging system makes it difficult to compare studies, leading to a lack of
advances in therapy or surveillance due to the rarity of these primary tumors.
Therefore, multicenter studies are necessary to explore non-surgical future
therapies that could be used as curative treatments for some patients.
Conflict of interest
Authors have no conflicts of
interest to declare.
Funding source
Personal
REFERENCES
1. Macchiarini
P. Primary tracheal tumours. Lancet
OnÂcol. 2006;7:83-91.
https://doi.org/10.1016/S1470-2045(05)70541-6.
2.
Chen F, Huang M, Xu Y, et al. Primary tracheal
adenoid cystic carcinoma: adjuvant treatment outcome. Int
J Clin Oncol. 2015;20:686-92. https://doi.org/10.1007/s10147-014- 0771-6
3. Ahn
Y, Chang H, Lim YS, Hah JH, Kwon TK, Sung MW, Kim KH. Primary tracheal tumors:
review of 37 cases. J Thorac Oncol. 2009;4:635-8.
https://doi.org/10.1097/JTO.0b013e31819d18f9
4. Wang SY, Wang SX, Liao JQ,
Chen G. 18°F-FDG PET/CT and Contrast-Enhanced CT of Primary Malignant Tracheal
Tumor. Clin Nucl Med. 2016
Aug;41:595-605. https://doi.org/10.1097/RLU.0000000000001228
5. Yang H, Yao F, Tantai J, Zhao Y, Tan Q, Zhao H. ReÂsected Tracheal Adenoid
Cystic Carcinoma: ImproveÂments in Outcome at a Single Institution. Ann Thorac Surg. 2016;101:294-300.
https://doi.org/10.1016/j.athoracÂsur.2015.06.073
6. Levy A, Omeiri
A, Fadel E, Le PĂ©choux C.
Radiotherapy for Tracheal-Bronchial Cystic Adenoid Carcinomas. Clin Oncol (R Coll
Radiol). 2018;30:39-46.
https://doi.org/10.1016/j.clon.2017.10.012
7. Zhao Y, Zhao H, Fan L, Shi J.
Adenoid cystic carcinoma in the bronchus behaves more aggressively than its
tracheal counterpart. Ann Thorac Surg. 2013;96:1998-2004.
https://doi.org/10.1016/j.athoracsur.2013.08.009
8. Calzada
AP, Miller M, Lai CK, Elashoff DA, Abemayor E, St John MA. Adenoid cystic carcinoma of the
airway: a 30-year review at one institution. Am J Otolaryngol. 2012;33:226-
31. https://doi.org/10.1016/j.amjoto.2011.07.003
9. Huston B, Froloff
V, Mills K, McGee M. Adenoid Cystic CarciÂnoma of the Trachea Resulting in
Fatal Asphyxia. J Forensic Sci. 2017;62:244-6.
https://doi.org/10.1111/1556-4029.13236
10. Hämetoja
H, Hagström J, Haglund C, Bäck L, Mäkitie A, Syrjänen S. Polyomavirus JCPyV infrequently detectable in adenoid cystic carcinoma
of the oral cavity and the airways. Virchows Arch.
2019;475:609-16.
https://doi.org/10.1007/s00428-019-02617-6
11.
Ministerio de Salud y Desarrollo Social de la NaciĂłn. InstiÂtuto Nacional del
Cáncer. (2019). EstadĂsticas-Mortalidad. Recuperado de:
https://www.argentina.gob.ar/salud/instituÂto-nacional-del-cancer/estadisticas/mortalidad
12. Doggett S, Chino S, Lempert T, Federhart J.
Percutaneous CT-fluoroscopic-guided radioisotope seed placement for the
management of adenoid cystic carcinoma of the trachea. Brachytherapy.
2017;16:639-45. https://doi.org/10.1016/j.brachy.2016.11.007
13. Kagotani
A, Ishida M, Yoshida K, Iwai M, Okabe H. Cytological
features of dedifferentiated adenoid cystic carcinoma of the trachea: a case
report. Diagn Cytopathol.
2014;42:880-3. https://doi.org/10.1002/dc.23064
14. Junker K. Pathology of
tracheal tumors. Thorac Surg
Clin. 2014;24:7-11.
https://doi.org/10.1016/j.thorÂsurg.2013.09.008
15. Garden AS, Weber RS, Morrison
WH, Ang KK, Peters LJ. The influence of positive
margins and nerve invaÂsion in adenoid cystic carcinoma of the head and neck
treated with surgery and radiation. Int J Radiat Oncol Biol
Phys. 1995;32:619-26. https://doi.org/10.1016/0360-3016(95)00122-F
16. Ishida M, Okabe H.
Dedifferentiated adenoid cystic carÂcinoma of the trachea: a case report with
respect to the immunohistochemical analyses of
mammalian target of rapamycin pathway proteins. Hum Pathol. 2013;44:1700-3.
https://doi.org/10.1016/j.humpath.2012.12.015
17. Webb BD, Walsh GL, Roberts
DB, Sturgis EM. Primary tracheal malignant neoplasms: the University of Texas
MD Anderson Cancer Center experience. J Am Coll Surg.
2006;202:237-46. https://doi.org/10.1016/j.jamcollÂsurg.2005.09.016
18. Jamjoom
L, Obusez EC, Kirsch J, Gildea
T, Mohammed TL. Computed tomography correlation of airway disease with
bronchoscopy--part II: tracheal neoplasms. Curr Probl Diagn Radiol.
2014;43:278-84.
https://doi.org/10.1067/j.cpradiol.2014.02.005
19. Gaissert
HA, Grillo HC, Shadmehr MB,
Wright CD, Gokhale M, Wain
JC, Mathisen DJ. Uncommon primary
tracheal tumors. Ann Thorac Surg. 2006;82:268-73. https://doi.org/10.1016/j.athoracsur.2006.01.065
20. Benissan-Messan
DZ, Merritt RE, Bazan JG, D'Souza DM, Abdel-Rasoul M, Moffatt-Bruce SD, Kneuertz
PJ. National Utilization of Surgery and Outcomes for PriÂmary
Tracheal Cancer in the United States. Ann Thorac
Surg. 2020;110:1012-22.
https://doi.org/10.1016/j.athoracÂsur.2020.03.048
21. Jiao W, Zhu D, Cheng Z, Zhao
Y. Thoracoscopic tracheal resection and
reconstruction for adenoid cystic carcinoma. Ann Thorac Surg. 2015;99:e15-7.
https://doi.org/10.1016/j.athoracsur.2014.06.118
22.
Sato K, Takeyama Y, y cols. Tracheal adenoid cystic carcinoma treated by repeated bronchoscopic argon plasma coagulation as a palliative
therapy. Ann Thorac Cardiovasc
Surg. 2014;20:602-5.
https://doi.org/10.5761/atcs.cr.12.02156
23. Himeji D, Kawaguchi T, Setoguchi K, Koreishi S.
Successful Treatment of Life-threatening Tracheal Stenosis Caused by Malignancy
with a Self-expanding Hybrid Stent. Intern Med. 2018;57:259-63.
https://doi.org/10.2169/internalmediÂcine.9164-17
24. Bittner N, Koh WJ, Laramore GE, Patel S,
Mulligan MS, Douglas JG. Treatment of locally advanced adÂenoid
cystic carcinoma of the trachea with neutron raÂdiotherapy. Int J Radiat Oncol
Biol Phys. 2008;72:410- 4.
https://doi.org/10.1016/j.ijrobp.2008.01.016 ijrobp.2008.01.016