Autor :Sousa MatĂas, Diego Alejandro1, González, Alejandra1, Idoyaga, Pablo1
1 Pulmonology Service Hospital Nacional Profesor Alejandro Posadas, Buenos Aires, Argentina
https://doi.org/10.56538/ramr.IZKD7855
Correspondencia : Diego Sousa. E-mail: diegosouÂsa88@hotmail.com
ABSTRACT
During the course of PBC, interstitial lung involvement may develop:
organizing pneumonia, interstitial fibrosis, lymphoid interstitial pneumonia,
or non-specific interstitial pneumonia. Although the diagnosis of PBC usually
precedes pulmonary manifestations, the opposite can occur. The frequency of
interstitial disease in patients with PBC is not exactly known. It may or may
not be associated with other connective tissue diseases; therefore, it is
necessary to carry out a systematic search of these diseases and the pulmonary
manifestations of this entity. We present the case of a patient with a previous
diagnosis of PBC, who developed interstitial lung involvement during the course
of the disease.
Key words: Primary biliary cholangitis, Interstitial lung
disease, Crazy paving
RESUMEN
Durante el transcurso de la colangitis biliar primaria se puede desarrollar
compromiso intersticial pulmonar: neumonĂa organizada, fibrosis intersticial,
neumonĂa intersticial linfoide, neumonĂa intersticial no especĂfica. A pesar de
que el diagnĂłstico de colangitis biliar primaria usualmente precede a las
manifestaciones pulmonares, puede ocurrir lo inverso. La frecuencia de
enfermedad intersticial en pacientes con colangitis biliar primaria no es
conocida con exactitud. Puede estar o no asociada a otras enfermedades del
tejido conectivo; por lo tanto, es necesario realizar una búsqueda sistemática
de estas y de las manifestaciones pulmonares de dicha entidad. Presentamos el
caso de una paciente con diagnĂłstico previo de colangitis biliar primaria, la
cual desarrolla durante el curso de su enfermedad, afectaciĂłn pulmonar
intersticial.
Palabras clave: Colangitis biliar
primaria, Enfermedad pulmonar intersticial, Crazy paving       Â
Received: 07/19/2023
Accepted: 08/16/2023
INTRODUCTION
Primary biliary cholangitis (PBC)
is an autoimÂmune, chronic, progressive disease of unknown etiology. It
predominantly affects middle-aged women and is characterized by cholestasis
caused by diffuse inflammation, destruction and fibrosis of intrahepatic bile
ducts, ultimately leading to cirrhosis, portal hypertension, and hepatic
failure.1
The diagnosis of PBC must meet at
least two of the following three criteria: 1) Chronic cholestasis with elevated
serum alkaline phosphatase and/ or gamma-glutamyl transpeptidase; 2) Presence of anti-mitochondrial
antibodies (AMA); and 3) Hepatic histopathological
characteristics indicaÂtive of PBC.2
Autoimmune diseases can be
observed in up to 84% of patients, with 41% having more than one concomitant
associated autoimmune condition.3,
4
PBC can also involve the
impairment of other organs, including the respiratory system.
There is limited literature
regarding the pulmoÂnary manifestations that can occur in patients with PBC,
and it is often challenging to distinguish lung involvement solely due to PBC
from that associÂated with another connective tissue disease. This is the
reason why the exact frequency of interstitial lung disease (ILD) is unknown.
Throughout the course of PBC,
several types of interstitial involvement can develop, including orÂganized
pneumonia, interstitial fibrosis, lymphoid interstitial pneumonia, non-specific
interstitial pneumonia, and granulomatous disease. These entities have been
described in different studies. Alveolar hemorrhage, airway obstruction, pulmoÂnary
hypertension, and pleural effusion can also be observed, though less
frequently.3 Despite the fact that the diagnosis of PBC usually precedes
pulmonary manifestations, the opposite can occur.5
We present the case of a patient
with a previous diagnosis of PBC, who developed interstitial lung involvement
during the course of the disease.
CASE REPORT
A 50-year-old female patient with
a history of smoking and diagnosed with PBC in 2017, along with portal
hypertension and computed tomograÂphy showing initial signs of bibasal interstitial disÂease, presented with a 3-day
history of abdominal distension and dyspnea.
Upon admission, the patient
exhibited hypoxÂemia, signs of ascites, and bibasal
crackling sounds. Initial laboratory tests revealed thrombocytopenia,
leukocytosis, elevated C-reactive protein, and norÂmal NT-proBNP
(N-terminal pro-brain natriuretic peptide) levels. The chest X-ray showed
bilateral alveolar infiltrates. The ascitic fluid
indicated preÂdominantly mononuclear cellular content.
The patient began antibiotic
therapy and treatÂment with diuretics, with a slow evolution of her condition.
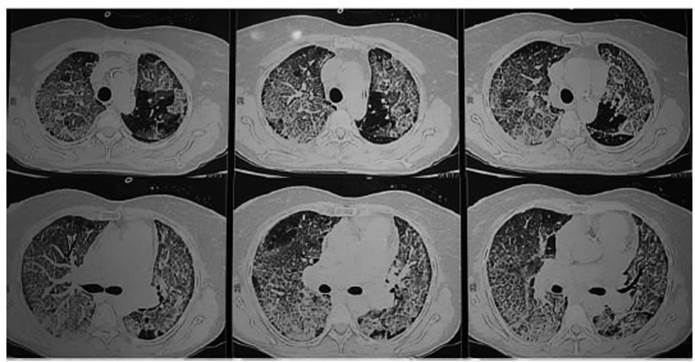
Subsequently, a chest CT scan was performed, revealing interstitial infiltrates
with a bilateral crazy-paving pattern (Figure 1). NasoÂpharyngeal swabs for
SARS-COV-2, mycoplasma, chlamydia, and influenza were all negative. The
immunological profile showed a positive antinucleÂar antibody (ANA) at a titer
of 1/320, positive anÂticentromere antibody, positive
anti-mitochondrial antibody, and elevated rheumatoid factor.
Systemic corticosteroid therapy
was initiated, leading to significant improvement in oxygen levÂels. However,
after 48 hours of noticeable clinical improvement, the patient experienced
upper gasÂtrointestinal bleeding and cardiac arrest. Despite resuscitation
efforts, the patient did not respond and passed away.
DISCUSSION
PBC is an autoimmune liver disease
characterized by the progressive destruction of intrahepatic bile ducts,
leading to cholestasis and fibrosis, which can ultimately result in cirrhosis
and hepatic failure.3
It predominantly affects women in
their fourth to sixth decades of life. Anti-mitochondrial antiÂbodies have high
specificity and are present in 90-95% of patients.6
Extrahepatic manifestations occur in over 70% of cases, mainly due to the
association with other autoimmune diseases such as Sjögren’s
syndrome (being the most commonly associated entity), hypo or hyperthyroidism,
systemic sclerosis, rheumatoid arthritis, and lupus.7
In a prospective study by Min Shen et al, which included 178 PBC patients from a Beijing
hospital between 2001 and 2007, it was observed that 84.4% of patients had an
association with other connecÂtive tissue diseases.8
There isn’t much data in the
literature regardÂing the prevalence of interstitial lung disease in patients
with PBC, but it is estimated to affect around 15% of the cases.9
In 1970, Mason et al published
the first report of interstitial lung disease in the course of PBC.10
In a retrospective study by Chen
et al, which included 136 patients with an average follow-up of 8.76 years from
1999 to 2014, they found that 11% of the cases had ILD.6
In a study by Wang et al,
involving a cohort of 332 patients in China, it was revealed that 46.6% had one
or more associated autoimmune diseases. The most frequent was Sjögren’s syndrome (121 cases, 36.2%). There were 9 cases
of systemic sclerosis (2.8%), 12 cases of systemic lupus eryÂthematosus
(3.7%), 9 cases of rheumatoid arthritis (2.8%), and 10 cases of polymyositis (3.1%). When compared to patients with PBC
alone, those with associated Sjögren’s syndrome or
systemic scleroÂsis had a higher frequency of ILD.11
In the study by Min Shen et al, where patients with conditions that could
confound the diagnoÂsis of ILD were excluded, they found that 15% of patients
had interstitial lung disease. While in most cases there was an association
with another connective tissue disease (mainly Sjögren’s
synÂdrome), 42.8% of the patients didn’t show any asÂsociation with another
condition. The risk factors that are mostly associated with the development of
ILD were: having an associated autoimmune disease and the Raynaud’s phenomenon.8
The most commonly observed CT
patterns in associated ILD during the course of PBC include reticular opacities
(39%), patchy opacities (25%), nodular opacities (25%), ground-glass
infiltrates (18%), interlobular septal thickening (18%),
and honeycombing (11%).9
It was believed that the
histological variant of fibrosis associated with PBC was similar to usual
interstitial pneumonia.12-14 Several reports describe that interstitial fibrosis, lymphoid
interstitial pneumonia (LIP), and organizing pneumonia are the most frequent
patterns in PBC. LIP can be associated with both PBC and Sjögren’s
synÂdrome. In a study by Sheng et al, lung biopsies were performed on 5
patients with ILD, revealing interstitial infiltrates predominantly composed of
lymphocytes, suggestive of LIP, in 3 patients. The other 2 biopsies were
compatible with interstitial fibrosis, vascular hyperplasia, and thickened
vascular walls.8 Organizing
pneumonia can be a manifestation of PBC, especially in patients with an
associated connective tissue disorder.15
Davison and Epstein reported a case of recurrent organizÂing
pneumonia in a patient with PBC, CREST syndrome, and chronic pancreatitis.16 However, it
can also occur in isolated cases of PBC. Almonte
Batista et al reported a case of a patient with PBC and organizing pneumonia,
without evidence of associated underlying connective tissue disorder.17
Among the differential diagnoses
for the crazy-paving pattern, the following should be considered: cardiogenic
or non-cardiogenic pulmonary edema, pneumonia (viral, bacterial, or fungal -
such as PCP [pneumocystis carinii
pneumonia]), alveolar hemorrhage, adult respiratory distress syndrome
(ARDS), vasculitis, and alveolar proteinosis,
among others. For proper characterization and distinction, it’s important to
separate acute causes from subacute or chronic ones.
Similarly, the etiolÂogy can be categorized based on infectious origins
(pneumonia), oncological causes, idiopathic facÂtors (organizing pneumonia, proteinosis, sarcoidÂosis, NSIP
[non-specific interstitial
pneumonia]), inhalation-related factors (hypersensitivity pneuÂmonitis,
lipid pneumonia), or blood-related factors (ARDS, alveolar hemorrhage
syndromes). 18-19
Information about the treatment
of ILD in the course of PBC is very limited. The response to agents like
corticosteroids and other immuÂnosuppressants can be
favorable. However, the recurrence rate is high, and unfortunately, cortiÂcosteroid
therapy doesn’t halt the progression of the liver disease.9
CONCLUSION
The frequency of interstitial
disease in patients with PBC is not exactly known. It may or may not be
associated with other connective tissue diseases; therefore, it is necessary to
carry out a systematic search of these diseases and the pulmonary maniÂfestations
of this entity.
In our patient, no clinical or
laboratory evidence of associated connective tissue disease or other
differential diagnoses with that CT pattern were found. Therefore, initially,
the ILD manifesting as a crazy-paving pattern corresponds to a pulmonary
manifestation specific to PBC.
Conflict of interest
Authors have no conflicts of
interest to declare.
REFERENCES
1. Franco I, Dubini A, Piciucchi
S, Casoni G, Poletti V.
Interstitial lung disease preceding primary biliary cirrhosis in a male
patient. Rev Port Pneumol. 2015;21:214-7.
https://doi.org/10.1016/j.rppnen.2015.02.008
2. European Association for the Study of the Liver. EASL Clinical
Practice Guidelines: management of cholestatic liver
diseases. J Hepatol. 2009;51:237-67. https://doi.org/10.1016/j.jhep.2009.04.009.
3. Koksal D, Koksal
AS, Gurakar A. Pulmonary Manifestations among
Patients with Primary Biliary Cirrhosis. J Clin Transl Hepatol.
2016;4:258-62.
https://doi.org/10.14218/JCTH.2016.00024.
4. Culp KS, Fleming CR, Duffy J, Baldus WP,
Dickson ER. Autoimmune associations in primary biliary
cirrhosis. Mayo Clin Proc. 1982;57:365-70.
5. Martusewicz-Boros MM, Boros
PW, Wiatr E. Respiratory system involvement in
chronic liver diseases. Pol Arch Med Wewn. 2013;123:635-42. https://doi.org/10.20452/pamw.1980.
6. Chen CT, Tseng YC, Yang CW, et al. Increased Risks of Spontaneous
Bacterial Peritonitis and Interstitial Lung Disease in Primary Biliary Cirrhosis
Patients With Concomitant Sjögren Syndrome. Medicine (Baltimore). 2016;95:e2537.
https://doi.org/10.1097/MD.0000000000002537.
7. Chalifoux SL, Konyn
PG, Choi G, Saab S. Extrahepatic Manifestations of
Primary Biliary Cholangitis. Gut Liver. 2017;11:771-80.
https://doi.org/10.5009/gnl16365.
8. Shen M, Zhang F, Zhang X. Primary biliary
cirrhosis complicated with interstitial lung disease: a prospective study in
178 patients. J Clin Gastroenterol. 2009;43:676-9.
https://doi.org/10.1097/MCG.0b013e31818aa11e.
9. Bartosiewicz M, Siemion-Szcześniak
I, Jędrych M, et al. Zmiany
śrĂłdmiąższowe w płucach
u chorych na pierwotną żĂłłciową
marskość wątroby
[Interstitial lung disease in patients with
primary biliary cirrhosis]. Pneumonol Alergol Pol. 2012;80:471-81. Polish. https://doi.org/10.5603/ARM.27559
10. Mason AM, McIllmurray
MB, Golding PL, Hughes DT. Fibrosing alveolitis associated with renal tubular acidosis. Br Med
J. 1970;4:596-9.
https://doi.org/10.1136/bmj.4.5735.596.
11. Wang L, Zhang FC, Chen H, et al. Connective tissue diseases in
primary biliary cirrhosis: a population-based cohort study. World
J Gastroenterol. 2013;19:5131-7.
https://doi.org/10.3748/wjg.v19.i31.5131.
12. Wallace JG Jr, Tong MJ, Ueki BH, Quismorio FP. Pulmonary involvement in primary biliary
cirrhosis. J Clin Gastroenterol. 1987;9:431-5.
https://doi.org/10.1097/00004836-198708000-00015.
13. Allan PF, Powers CR, Morris MJ. Pulmonary
manifestations of primary autoimmune hepatobiliary
disease. Clin Pulm
Med 2005;12:232-45. https://doi.org/10.1097/01.cpm.0000171500.70809.d1.
14. Osaka M, Aramaki T, Okumura H, Kawanami O. Primary biliary cirrhosis with fibrosing alveolitis. Gastroenterol Jpn.
1988;23:457-60. https://doi.org/10.1007/BF02779216.
15. Harada
M, Hashimoto O, Kumemura H, et al. Bronchiolitis obliterans organizing pneumonia
in a patient with primary biliary cirrhosis and rheumatoid arthritis treated
with prednisolone. Hepatol Res. 2002;23:301. https://doi.org/10.1016/s1386-6346(02)00006-2.
16. Davison AG, Epstein O. Relapsing organising
pneumonitis in a man with primary biliary cirrhosis, CREST syndrome, and
chronic pancreatitis. Thorax. 1983;38:316-7.
https://doi.org/10.1136/thx.38.4.316.
17. Almonte Batista WM, Sánchez
SimĂłn-Talero R, Almonte GarcĂa CE, Núñez Ares A, AgustĂn MartĂnez FJ, GarcĂa
Guerra JA. Cirrosis biliar primaria con neumonĂa organizada secundaria. Rev SOCAMPAR. 2017;2:67-9.
18. De Wever
W, Meersschaert J, Coolen
J, Verbeken E, Verschakelen
JA. The crazy-paving pattern: a radiological-pathological
correlation. Insights Imaging [Internet].
2011;2:117-32. https://doi.org/10.1007/s13244-010-0060-5
19.
Rossi SE, Erasmus JJ, Volpacchio
M, Franquet T, Castiglioni
T, McAdams HP. “Crazy-Paving” Pattern at Thin- Section CT of the Lungs: Radiologic-Pathologic
Overview. RadioGraphics [Internet].
2003;23:1509-19. https://doi.org/10.1148/rg.236035101