Autor :Carmona, Alejandro A.1,4, Carmona, Juan M.2,3,4, Abdala, Jorge A.2,3,4
1 Surgical Service. Hospital Luis Lagomaggiore, Mendoza, Argentina.
2 Thoracic Surgery. ClĂnica de Cuyo. Mendoza, Argentina.
3 Thoracic Surgery. Hospital Santa Isabel de HungrĂa, Mendoza, Argentina.
4Thoracic Surgery. ClĂnica Santa MarĂa, Mendoza, Argentina.
https://doi.org/10.56538/ramr.GTQJ4007
Correspondencia : Alejandro A. Carmona. Mail: alecarmonab@hotmail.com
ABSTRACT
Centuries
after the detection of amyloid material as extracellular deposits, we find
various classifications in the literature. Regarding thoracic involvement, four
types are recognized, with the tracheobronchial type being the least common.
We
present the case of a 32-year-old male patient with no previous medical record,
who sought medical attention due to a 3-year history of dysphonia. A nodular
lesion was found in the posterior wall of the trachea. A biopsy was performed
through fiberoptic bronchoscopy, and the result indicated the presence of
tracheobronchial amyloidosis. Chemotherapy was administered, and the
possibility of surgical resection was evaluated basing on treatment response.
Pulmonary
amyloidosis associated with systemic amyloidosis typically presents as a
diffuse interstitial pattern. Tracheobronchial and nodular parenchymal forms
are unÂcommon and manifest with obstructive symptoms. A definitive diagnosis
was achieved through biopsy. There are various therapeutic options available,
such as chemotherapy, autologous bone marrow transplantation, and for patients
with obstructive symptoms, endoscopic resection and stent placement are
recommended.
Key words:
Amyloidosis;
Computed tomography; Treatment
RESUMEN
Siglos
después de la detección de material amiloide como
depósitos extracelulares, encontramos en la bibliografía diversas
clasificaciones. En cuanto a la afección torácica, se reconocen
cuatro tipos; el traqueobronquial es el menos frecuente.
Presentamos
el caso de un paciente masculino de 32 años sin antecedentes, que
consulta por disfonía de 3 años, al que se le constata una
lesión nodular en la pared posterior de la tráquea. Se realiza una
biopsia por fibrobroncoscopia, cuyo resultado indica la presencia de
amiloidosis traqueobronquial. Se realiza quimioterapia y se valora la
realización de una resección quirúrgica según
respuesta al tratamiento.
La
amiloidosis pulmonar asociada con amiloidosis sistémica generalmente se
presenta como un patrón intersticial difuso. Las formas traqueobronquial
y parenquimatosa nodular no son frecuentes y se manifiestan con síntomas
obstructivos. El diagnóstico definitivo se logra mediante biopsia.
Disponemos de diversas opciones terapéuticas, como la quimioterapia, el
trasplante autólogo de médula ósea y, para pacientes con
síntomas obstructivos, se recomienda la resección
endoscópica y colocación de stent.
Palabras clave:
Amiloidosis;
Tomografía computada; Tratamiento
Received: 02/13/2023
Accepted: 03/06/2023
INTRODUCTION
In
1854, Rudolph Virchow coined the term “amyÂloid” to describe the presence of
extracellular deposits of a substance similar to cellulose that reacted to
iodine. More than 150 years later, the term “amyloidosis” is used to describe
the disÂease characterized by deposits of protein fibers of amyloid material in
the tissues, caused in part by imperfect protein metabolism. This progressive
accumulation of material that is insoluble and resistant to proteolytic
metabolism causes slow and progressive damage to the affected organs, leading
to functional collapse.1-3
In
the global literature, multiple classifications are described for this disease
based on its relationÂship with a triggering cause, histological types,
affected organs, or the type of protein deposited.3
Four
types of thoracic amyloidosis with different characteristics are recognized and
can be identified by computed tomography (CT): tracheobronchial, nodular
parenchymal, diffuse parenchymal, and lymphadenopathy.2
Tracheobronchial
involvement, either isolated or in the context of a systemic disease is an
unusual form of presentation, accounting for approximately 1% of benign lesions
in the tracheobronchial tree. All of this, together with the limited
information available in the global literature, poses a diagnostic challenge
for physicians.4
We
report the case of a patient treated by our team with a diagnosis of
symptomatic tracheobronÂchial amyloidosis in the context of asymptomatic
systemic disease.
CASE REPORT
32-year-old
male patient. No medical history. The patient presented with a 3-year history
of dysÂphonia without any other associated symptoms. Rhinoscopy and
laryngoscopy were performed, revealing a cystic edema-like lesion located in
the anterior third of the right ventricle, vocal cord edeÂma, and enlarged and
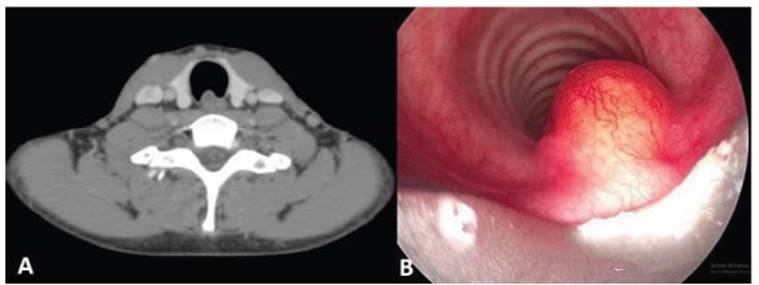
irregular ventricular bands. A computed axial tomography (CAT) showed an 8 mm Ă—
10 mm Ă— 15 mm lesion in the posterior wall of the trachea, at the level of the
thyroid. This lesion was nodular, slightly hypodense, of non-specific nature,
and didn’t change after the administration of intravenous contrast nor during
the phonation cycle.
Also,
small hypodense images measuring 2.7 mm and 3.7 mm were observed at the free
edge of the right inferior vocal cord, and irregular border images were found
in the left inferior vocal cord, not exceeding 10 mm in size (Figure 1A). With
these results and due to the persistence of the patient’s dysphonia, a rigid
fiberoptic bronchosÂcopy (FBC) was performed, revealing a nodular, sessile
lesion in the trachea with growth towards the lumen. A biopsy of the described
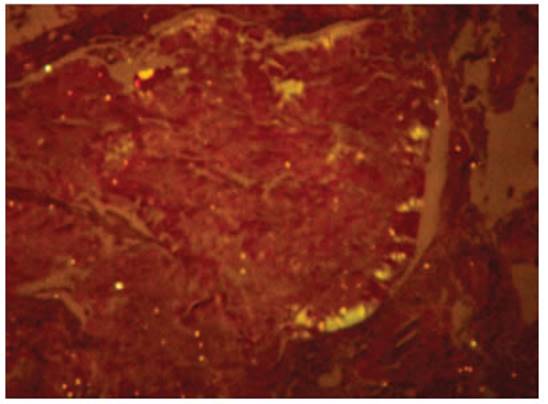
lesion was performed (Figure 1B). The histopathological reÂport revealed
laryngeal mucosa without dysplastic changes. There was infiltration of the
chorion by a hypocellular, amorphous eosinophilic tissue that exhibited Congo
red birefringence under polarÂized light. Few multinucleated giant cells were present,
and there were no signs of vasculitis. These findings are consistent with
amyloidosis (Figure 2). It was also decided to perform a biopsy of abdominal
adipose tissue and bone marrow to evaluate primary systemic amyloidosis, which
yielded a positive result despite the fact that the patient didn’t present any
signs or symptoms of systemic involvement. The requested laboratory analysis
did not show relevant alterations. The cardiac nuclear magnetic resonance
didn’t show any sign of cardiac amyloidosis. The proposed treatment consisted
of chemotherapy cycles with bortezomib in combination with low-dose dexaÂmethasone,
along with symptomatic treatment provided by specialists in Speech Therapy and
Otorhinolaryngology.
DISCUSSION
The literature highlights a higher incidence of amyloidosis in male patients over 50 years of age, but respiratory system involvement, particularly in the form of nodules in the tracheobronchial tree, is rare.
There
are few published reports on cases of localized primary tracheobronchial
amyloidosis, and few authors have described cases of systemic amyloidosis
including thoracic involvement in the form of nodules in the airway and
respiratory symptoms exclusively.5 The presented
case aligns with the predominant gender, but corresponds to a much younger age
range than the one typical for the diagnosis of this disease. It is noteworthy
that the onset of symptoms and disease appearance correspond to pulmonary
involvement, and the systemic involvement in the absence of symptoms of other
systems is a finding of interest.
In
a retrospective study conducted at Mayo Clinic evaluating patients with
pulmonary amyÂloidosis treated at their center over a period of 13 years, it was
concluded that out of the 55 included patients 35 had primary systemic
amyloidosis with pulmonary involvement, 17 had localized pulmoÂnary
amyloidosis, and 3 had secondary familial amyloidosis. They established that
pulmonary amyloidosis associated with primary systemic amyÂloidosis generally
presents as a diffuse interstitial pattern with or without pleural effusion.6
Another
more recent study from Mayo Clinic states that patients with localized
pulmonary amyÂloidosis generally don’t show evidence of systemic amyloidosis
and only have isolated involvement of the parenchyma or the tracheobronchial
system.7 This highlights
the uniqueness of the case treated by the authors, where, in the absence of any
pathoÂlogical history, the patient presented with mild upper airway symptoms,
without involvement of the pulmonary interstitium or pleura, and only a nodular
formation was found in the posterior wall of the trachea, which tested positive
for amyloidoÂsis on biopsy. The patient was thoroughly studied, and a positive
result for amyloidosis was obtained from a biopsy of adipose tissue.
Isolated
tracheobronchial amyloidosis has a variable presentation, ranging from
asymptomatic to common symptoms, including dysphonia, striÂdor, dyspnea, cough,
hemoptysis, and dysphagia, with significant morbidity and mortality due to
obstructive phenomena. It has subacute preÂsentation and typically begins with
progressive dyspnea, wheezing, cough, pneumonia, and epiÂsodes of hemoptysis.
In cases of tracheobronchial and nodular parenchymal forms, the symptoms
generally depend on the segment of the respiraÂtory tree that is affected.
Patients with proximal airway involvement predominantly experience obstructive
symptoms. On the contrary, those with lesions in the middle airway will experience
more symptoms derived from lobar collapse and recurrent infections, while cases
where the distal airway is affected may show a history of recurrent pneumonia
and bronchiectasis.6, 8 It is important
to highlight the fact that the only symptom of the patient that was treated by
the attending team, which led him to seek medical attention, was a 3-year
history of dysphonia without any other asÂsociated symptoms, which is uncommon
according to the literature consulted.
Many
cases are initially diagnosed as bronchial asthma, leading to the prescription
of incorrect treatments. When symptoms persist, further inÂvestigation is
necessary to achieve the diagnosis of respiratory amyloidosis.9
While a definitive diagnosis is made through the histopathological
result of a biopsy stained with Congo red under polarized light, the chest CAT
scan and FBC are effective complementary tests with high sensitivity and
specificity for tracheobronchial amyloidosis.10
In the present case, the diagnosis was achieved through CAT scan
and biopsy performed through FBC.
A
multicenter German study, which represents the largest cohort in the
literature, investigated the use of chest CAT scans in patients with variÂous
presentations of respiratory amyloidosis. The study describes the different
findings that can be observed in these patients and the significant differences
between those with tracheobronchial amyloidosis and those with parenchymal
variants, either the nodular or the diffuse type. In the first circumferential
thickenings, with a reduction in the tracheal lumen, localized wall thickening,
and tracheal calcifications were found.11
Currently,
there is a wide range of therapeutic options available for systemic
amyloidosis. One opÂtion is conventional chemotherapy with low doses of
dexamethasone or its combination with melphaÂlan. Favorable results have also
been reported with the combination of proteasome inhibitors, such as
bortezomib, in therapy cycles like CYBORD (cyclophosphamide, bortezomib,
dexamethasone). This, combined with an autologous bone marrow transplantation,
has achieved disease control in over 65% of patients. The use of iodinated
anthraÂcycline drugs, such as doxorubicin, has yielded favorable results as it
binds to amyloid protein fibers, promoting their degradation.12
Regarding
the treatment of amyloid deposits in the tracheobronchial tree in the form of
tumor-like growth with involvement of the airway lumen, the therapeutic
approach will depend on the severÂity of the patient’s respiratory symptoms. Good
initial results are described with pharmacological treatment or external beam
radiation therapy in patients with mild signs and symptoms, achieving symptom
control within a month and up to five years, as described by Neben-Wittich from
Mayo Clinic in their published case report. For patients experiencing symptom
progression due to mass growth or initially presenting with symptoms that
impair their quality of life, endoscopic laser resection is recommended. A
thoracic surgery team in Naples, Italy, has published a case in which a patient
underwent endoscopic laser photoresection of a mass that compromised 80% of the
tracheal lumen and left bronchus. Subsequently, a self-expandable stent was
placed to preserve airway permeability in the event of recurrence or
scar-related airway stenosis.13-15 Considering
that the patient had mild symptoms without progression in recent years and no
impact on quality of life, taking into account that the tracheobronchial inÂvolvement
is associated with systemic amyloidosis, a decision was made to initiate
chemotherapy with bortezomib and dexamethasone. The patient also received
phoniatric therapy from the Speech TherÂapy and Otorhinolaryngology Department
and underwent regular follow-up evaluations together with symptom evaluation
and blood count test to assess treatment response. Autologous bone marÂrow
transplantation was to be considered in case of therapeutic failure. 15 months
after diagnosis the patient was asymptomatic, in good clinical condition, and
showed good treatment response.
Conflict of interest
Authors
have no conflict of interest to declare.
REFERENCES
1.
Medina Castillo DE , Quiroz Mejía R, Caliope
Carrera E, et al. Amiloidosis sistemática. Dermatol Rev Mex. 2015;208-
18.
2.
Aylwin AC, Gishen P, Copley SJ. Imaging appearance of thoracic amyloidosis. J
Thorac Imaging. 2005;20:41-6.
https://doi.org/10.1097/01.rti.0000154074.29194.09
3.
Lado Lado FL, Ferreiro Regueiro MJ, Cabana Gonzalez B, Diez Diez V, Maceda
Vilariño S, Antunez Lopez JR. Amiloidosis. Medicina Integral. 2000;36.
4.
Peng X, Wang X, Luo D, Zuo W, Yao H, Zhang W. Atypical primary pulmonary
amyloidosis: A rare case report. MediÂcine (Baltimore). 2020;99:e20828.
https://doi.org/10.1097/MD.0000000000020828
5.
Piazza C, Cavaliere S, Foccoli P, Toninelli C, Bolzoni A, Peretti G. Endoscopic
management of laryngo-tracheoÂbronchial amyloidosis: a series of 32 patients.
Eur Arch Otorhinolaryngol. 2003;260:349-54.
https://doi.org/10.1007/ s00405-003-0592-0
6.
Utz JP, Swensen SJ, Gertz MA. Pulmonary amyloidosis. The Mayo Clinic experience
from 1980 to 1993. Ann Intern Med. 1996;124:407-13.
https://doi.org/10.7326/0003-4819-124-4-199602150-00004
7.
Capizzi SA, Betancourt E, Prakash UB. Tracheobronchial amyloidosis. Mayo Clin
Proc. 2000;75:1148-52. https://doi.org/10.4065/75.11.1148
8.
Birkeland AC, McHugh JB, Spector ME. Tracheobronchial amyloidosis: A case
report and review of the literature. J Case Rep Med. 2014;3:235859.
https://doi.org/10.4303/jcrm/235859
9.
Milani P, Basset M, Russo F, Foli A, Palladini G, Merlini G. The lung in
amyloidosis. Eur Respir Rev. 2017;26:170046.
https://doi.org/10.1183/16000617.0046-2017
10.
Tanrıverdi E, Özgül MA, Uzun O, et al. Tracheobronchial
Amyloidosis Mimicking Tracheal Tumor. Case Rep Med. 2016;2016:1084063.
https://doi.org/10.1155/2016/1084063
11.
Brandelik SC, Heussel CP, Kauczor HU, et al. CT feaÂtures in amyloidosis of the
respiratory system - ComÂprehensive analysis in a tertiary referral center
cohort. Eur J Radiol. 2020;129:109123.
https://doi.org/10.1016/j.ejrad.2020.109123
12.
Ryšavá R. AL amyloidosis: advances in diagnostics and treatment.
Nephrol Dial Transplant. 2019;34:1460-6.
https://doi.org/10.1093/ndt/gfy291
13.
Sommer P, Kumar G, Lipchik RJ, Patel JJ. TracheobronÂchial amyloidosis managed with
multimodality theraÂpies. Ther Adv Respir Dis. 2014;8:48-52.
https://doi.org/10.1177/1753465814524470
14.
Fiorelli A, Accardo M, Galluccio G, Santini M. Amiloidosis traqueobronquial
tratada con resección con láser endoÂbronquial y prótesis
en Y autoexpansible. Arch Bronc. 2013;49:303-5.
https://doi.org/10.1016/j.arbres.2012.11.013
15.
Dahl KA, Kernstine KH, Vannatta TL, Karwal MW, Thomas KW, Schraith DF.
Tracheobronchial amyloidosis: a surgical disease with long-term consequences. J
Thorac Cardiovasc Surg. 2004;128:789-92.
https://doi.org/10.1016/j.jtcvs.2004.03.036