Autor : Garza-Beltrán, Marco Antonio1, Flores-Hernández, Karen HerĂ©ndira1, Belalcazar, VĂctor Manuel2, PĂ©rez de LeĂłn Vázquez, Martha Patricia3, González de la Parra, Mario4, Delgado-Roche, Livan5
1Instituto de Investigaciones Aplicadas a la Neurociencia. A.C., Clinical Research, Durango, Durango, Mexico.
2 ĂŤcaro Investigaciones en Medicina. S.A. de C.V., Clinical Research, Chihuahua, Chihuahua, Mexico.
3Hospital Mdica Sur, Otolaryngology Service, City of Mexico, Mexico.
4Biokinetics S.A. de C.V.
5Laboratorios Liomont, S.A. de C.V. 8
https://doi.org/10.56538/ramr.DICZ6213
Correspondencia : Livan Delgado-Roche: E-mail: ldelgado@liomont.com.mx
ABSTRACT
Objective: The objective of the present study was to evaluate the efficacy and
safety of the fixed dose combination of montelukast/desloratadine 10 mg/5 mg
capsule versus the combination of montelukast/loratadine 10 mg/10 mg tablet in
adults diagnosed with persistent allergic rhinitis.
Materials and methods: The present study was a multicenter, controlled, prospective,
longitudinal, randomized, double-blind clinical trial with parallel arms.
Patients diagnosed with persistent allergic rhinitis who
met eligibility criteria and signed informed consent were enrolled in the study
to receive one of the two treatments every 24 hours orally for 6 weeks.
Efficacy was established by clinical evaluation through clinical scales validated
in Spanish, being the primary efficacy variable the difference in the score of
the SNOT-20 (Sino-Nasal Outcome Test) questionnaire at the end of treatment;
and the frequency and characteristics of adverse events were considered the
safety variable.
Results: 86 patients were randomized, 74 of which were analyzed per protocol.
Questionnaires about the symptoms of the disease and quality of life
indicators with both treatments showed that more than 90% of patients had mild
symptoms or no symptoms at all at the end of the study. So, both treatments
significantly improved (p < 0.05) the symptoms of the disease. Adverse
events were mild to moderate.
Conclusions: The present study showed that the efficacy of montelukast/desloratadine
10 mg/5 mg is not inferior to the comparator. Therefore, the study treatment
represents an effective and safe alternative for the second-line treatment of
persistent allergic rhinitis in patients in whom monotherapies or first-line
treatments dont offer clinically relevant improvement.
Key words: Montelukast, Desloratadine, Loratadine, Allergic rhinitis
RESUMEN
Objetivo: El objetivo del presente estudio fue evaluar la eficacia y seguridad de la
combinación de dosis fija montelukast/desloratadina 10mg/5mg
cápsula versus la combinación de montelukast/loratadina 10 mg/10
mg tableta en adultos con diagnóstico de rinitis alérgica persistente.
Material y métodos: El presente fue un estudio clínico aleatorizado, controlado, doble
ciego, prospectivo, longitudinal, multicéntrico, con brazos paralelos.
Sujetos con diagnóstico de rinitis alérgica persistente que
cumplieran criterios de elegibilidad y firmaran consentimiento informado fueron
enrolados para recibir uno de los dos tratamientos cada 24 horas vía
oral durante 6 semanas. La eficacia se estableció mediante la
evaluación clínica a través de escalas clínicas
validadas en idioma español, siendo la variable primaria de eficacia la
diferencia de puntuación del cuestionario SNOT-20 al final del
tratamiento, mientras que la frecuencia y características de los eventos
adversos fue considerada la variable de seguridad.
Resultados: Se aleatorizaron 86 pacientes, 74 de ellos fueron analizados por protocolo.
Los cuestionarios sobre síntomas de la enfermedad e indicadores de
calidad de vida con ambos tratamientos mostraron que más del 90% de los
pacientes no presentaron síntomas o solo fueron leves al final del
estudio, por lo que ambos tratamientos mejoraron significativamente (p <
0.05) la sintomatología de la enfermedad. Los eventos adversos
presentados fueron leves a moderados.
Conclusiones: El presente estudio demostró que la eficacia de montelukast/desloratadina
10 mg/5 mg no es inferior al medicamento comparador. Por tanto, el tratamiento
de prueba representa una alternativa eficaz y segura para el tratamiento de
segunda línea de la rinitis alérgica persistente en pacientes que
las monoterapias o primeras líneas de tratamiento no ofrecen
mejoría clínicamente relevante.
Palabras clave: Montelukast, Desloratadina, Loratadina, Rinitis alérgica
Received: 28/07/2022
Accepted: 07/12/2022
INTRODUCTION
Allergic rhinitis (AR) is a disease
that affects around 40% of the world population, whereas Mexico reports an
estimate of total prevalence of 15%.1, 2 The two most common
symptoms that most strongly affect the patients quality of life are rhinorrhea
and nasal congestion. Half of the patients with AR in Mexico have persistent
rhinitis, and the congestive component is present in almost 90% of the
patients.3
AR can be classified as
persistent when the symptoms occur 4 or more days a week or during 4 or more
weeks.4 Moderate to
severe symptoms affect the patients capacity to do daily activities and are
associated with fatigue, changes in the patients mood, cognitive disorders,
depression and anxiety.5 AR treatment
requires preventive measures such as avoiding contact with the allergen as much
as possible or, the most common treatment, pharmacotherapy.6
In that sense, the ARIA (Allergic Rhinitis and its Impact on
Asthma) Guidelines recommend the use of intranasal corticosteroids and second-generation
anti-histamines as first-line treatment, as well as the use of
anti-leukotrienes or immunotherapy, when Persistent Allergic Rhinitis PAR
doesnt respond to primary treatment.4, 7, 8
Montelukast is an
anti-leukotriene that binds with high affinity and selectivity to the
cysteinyl-leukotriene receptor 1 (CysLTR-1), thus inhibiting the physiological
actions of leukotrienes C4, D4 and E4, directly associated with the symptoms of
AR.9, 10 Desloratadine,
on the other hand, is a second-generation antihistamine, selective antagonist
of histamine H1 receptors. It doesnt penetrate the central nervous system and
has high affinity for such receptor compared to cetirizine, ebastine,
loratadine and fexofenadine; in addition, desloratadine has a longer half-life
(27 h), which produces a substantial benefit in nasal and ocular symptoms in
patients with moderate AR as opposed to other second-generation antihistamines.11-13
The combination of these two
drugs is a comprehensive treatment for the allergic process; it is aimed at
different molecular targets within the physiopathological process of PAR. The
therapeutic effects of desloratadine theoretically have advantages over
loratadine, since it is considered the active metabolite of the drug. Also,
given the fact that this is a convenient treatment (only 1 time a day), it can
contribute to patient compliance and successful pharmacotherapy.
Even though there is evidence on
the efficacy and safety of this combination for PAR14,15,22,23, it is not available in the
Mexican market, so it is necessary to show the efficacy and safety prior to
requesting sanitary registration from the regulatory authority. So, the
objective of this study was to evaluate the efficacy and safety of the
fixed-dose combination of montelukast/desloratadine 10 mg/5 mg in comparison
with montelukast/ loratadine 10 mg/10 mg administered once a day for 6 weeks.
MATERIALS AND METHODS
Study design and population
Controlled, randomized,
double-blind, therapeutic confirmatory, prospective, longitudinal,
parallel-group, multicenter clinical trial including Mexican adult patients
diagnosed with PAR of at least one year of evolution with moderate to severe
signs and symptoms according to the ARIA classification, and a baseline SNOT-20
score of at least 3 points. Exclusion criteria: patients with history of
asthma, hypersensitivity to any of the study drugs or formulation excipients,
recent respiratory infections, history of rhinosinusitis, problems with nasal
structures, including nasal polyps, septum deviation (around 70%) that
significantly impact the nasal airflow, patients addicted to steroids or
decongestant inhalers, pregnant or lactating women, use of acetylsalicylic acid
or concomitant use of immunotherapy or antihistamines that couldnt complete an
elimination lavage period of at least 7 half-lives before enrollment.
Patients were enrolled after they
signed their informed consent. The protocol and every document that has been
delivered or applied to patients were previously approved by Research Ethics
Committees and Research Committees in accordance with the local rules. All the
procedures were performed in accordance with the Declaration of Helsinki and
Good Clinical Practice (ICH E6R2).
Research centers were distributed
in different states of the Mexican Republic, including the Otolaryngology
Service of the Hospital Médica Sur (City of Mexico, Mexico), the
Instituto de Investigaciones Aplicadas a la Neurociencia, A. C. (Durango,
Mexico), and Ícaro Investigaciones en Medicina, S.A. de C.V. (Chihuahua,
Mexico).
Treatments
Research subjects were randomized
at a 1:1 ratio to the treatment arm with the study drug
montelukast/desloratadine 10 mg/5 mg capsules or to the comparator arm with
montelukast/loratadine 10 mg/10 mg tablets (Montaclar), both treatments administered every 24 hours
(evening dose) orally for 6 weeks.
Study variables
Treatment efficacy was determined
through the global score of the SNOT-20 (Sino-Nasal Outcome Test) questionnaire,16
and also through information collected from medical history, physical
exploration with previous rhinoscopy, and the scores of the T5SS (Total
5-Symptom Score),17 and TSQM (Treatment Satisfaction Questionnaire
for Medication) questionnaires.18 The tests were conducted during
baseline assessment (day -7), at the start of treatment (day 1), on day 21
(follow-up) and at the end of treatment (day 42).
The primary efficacy variable was
established as the difference between the baseline global score of the SNOT-20
questionnaire and the global score obtained in week 6. If the difference
between the baseline and final score is more than zero (positive), it is
interpreted as a favorable result, but if the value is less than zero, it is
interpreted as an unfavorable result. A change of more than 3 points in the
global score of SNOT-20 was considered an improvement of clinical relevance.
Secondary efficacy variables are
the area under the curve (AUC) of the SNOT-20 of each visit, SNOT-20 indicators
per treatment and per visit, the severity classification of SNOT-20 scores, the
T5SS questionnaire, T5SS indicators per treatment and per visit (21 days and 42
days), severity classification of T5SS scores, use of the rescue drug (inhaled
mometasone, prohibited during the first 10 days, use allowed for 2 weeks,
maximum), and also the scores of the TSQM questionnaire.
Statistical analysis
Sample size was calculated taking
into account the standard deviation (SD) of 1 point in the SNOT-20
questionnaire reported by Piccirillo et al, 200219
and a delta of 0.8, which is considered clinically significant by
the same author. The sample was calculated with the PASS 13 program, also
considering a significance level of 2.5% and 90% power for the non-inferiority
hypothesis. Taking into account 20% of withdrawals, the sample was established
in 86 patients.
The statistical analysis used the
Students t Test or the Mann-Whitney U Test for the comparison of mean
values. For the analysis of the variables on a categorical scale (nominal or
ordinal), the Fishers Exact Test was used. Demographic and baseline
characteristics are presented with descriptive statistics. The statistical
analysis was carried out using the Stata
15 (StataCorp, Texas, United States), NCSS
11 (NCSS, LLC. Kaysville, Utah, United States)
and East version 6
(Cytel Inc, United States) programs. The significance level for variable
analysis was set at 5% (Type I error, α = 0.05), except for the non-inferiority test, whose level of
significance was set at 2.5% (Type I error, α = 0.025) for being unilateral.
RESULTS
In the present study, 44 patients
were enrolled for the group that received treatment with the active comparator,
MKLOR (montelukast/loratadine), and 42 patients were included in the test
group, MKDES (montelukast/desloratadine), for a total of 86 patients randomized
for the intention-to-treat (ITT) population. During the database review, under
double-blind conditions, subjects with baseline SNOT-20 scores < 3 (visits
on day -7 and day 1) were discarded, according to the eligibility criteria,
leaving 37 patients in each group for a total of 74 individuals in the per
protocol population (Figure 1).
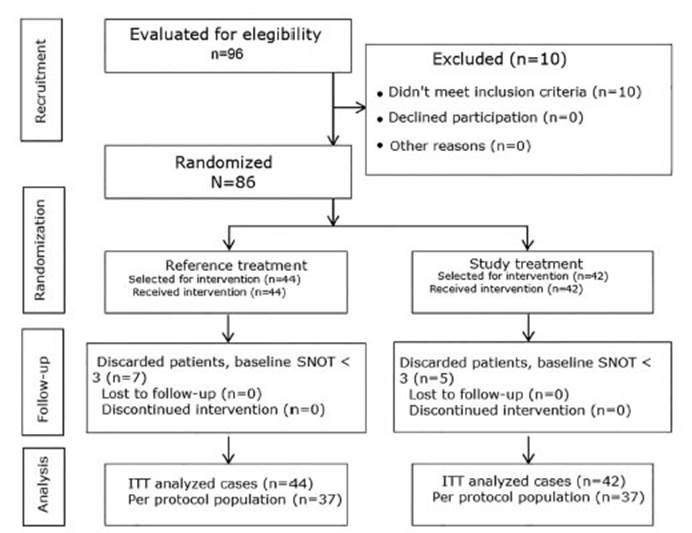
The per protocol population
(n=74) allowed the evaluation of efficacy variables (primary and secondary),
whereas in the intention-to-treat population (n=86), the demographic and
clinical variables, the TSQM questionnaire, and safety and tolerability
variables were evaluated for the confirmatory analysis of the primary efficacy
variable.
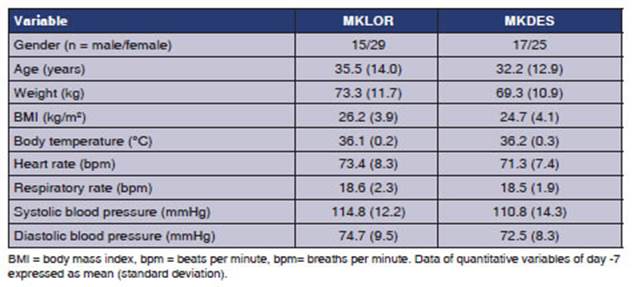
62.8% (n=54) of the 86 patients
were female, however, the demographic variables and data from medical records
(vital signs) didnt show clinically relevant differences between treatment
groups (Table 1).
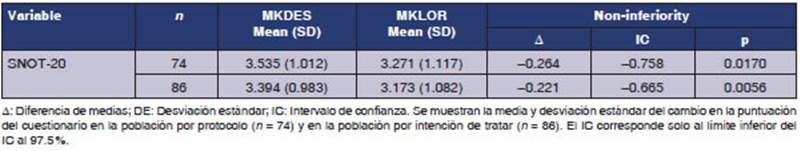
The primary efficacy analysis
showed that the change in the global score of the SNOT-20 questionnaire was
more than 3 points for both groups in the per protocol population, with a value
of 3.54 points in those treated with MKLOR (-0.78 to 4.80) and 3.27 points
(0.03 to 4.35) for MKDES; the difference in means (test-reference) was -0.26
points, with a 97.5% CI lower limit of -0.76 points, not exceeding the
clinically relevant inferiority margin of -0.8 (p=0.0170).19 Therefore, treatment with MKDES is not
inferior to MKLOR in terms of efficacy for the treatment of PAR symptoms. This
was verified by the ITT population (p=0.0056), with a difference of means of 0,22 points and a 97,5% CI lower limit of 0,67 points (Table
2).
The potential impact of the
demographic variables was evaluated by linear regression, considering the
change in the global SNOT-20 score as the dependent variable, and the
treatment, research site, age, gender, and body mass index as independent
variables. Only the research site had a significant effect on the primary
efficacy variable (p <0.0001). Individuals from one center had a smaller
change in score compared to the other centers. Since this occurred in only 10
patients, it was not considered to have a significant impact on the conclusion
of the non-inferiority test, and was considered a common finding of multicenter
studies.
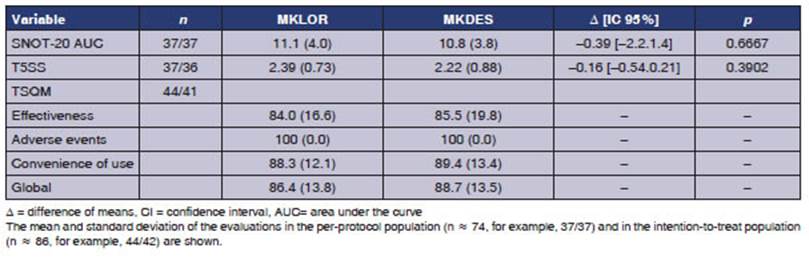
In the secondary efficacy
analysis, global scores from the baseline measurement to the last week showed a
mean difference of -0.393 units of area under the curve, with no significant
difference between the groups (p = 0.6667). No significant difference was found
for the global change of the T5SS (p = 0.3902), which is consistent with the
conclusion of on-inferiority of the primary efficacy variable. The global TSQM
score was greater than 80% in both groups, as were the dimensions of
effectiveness and convenience of use; the adverse events dimension suggested a
high degree of tolerability (Table 3).
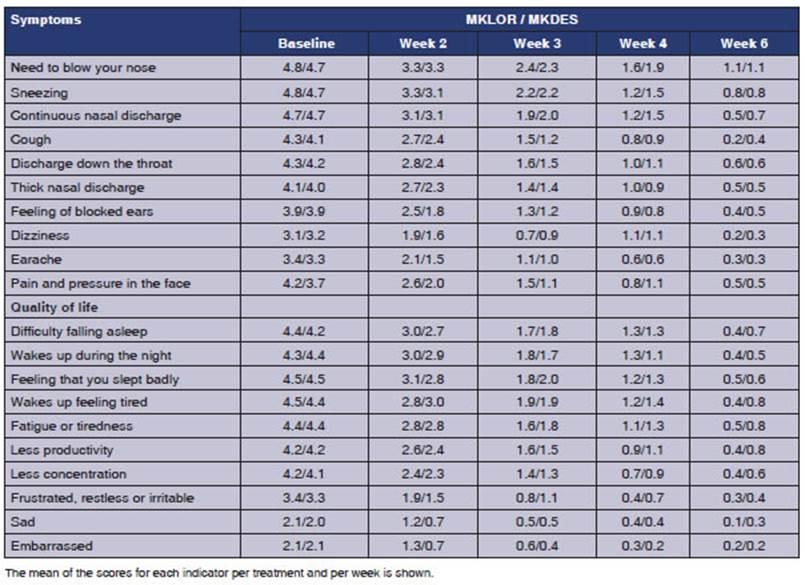
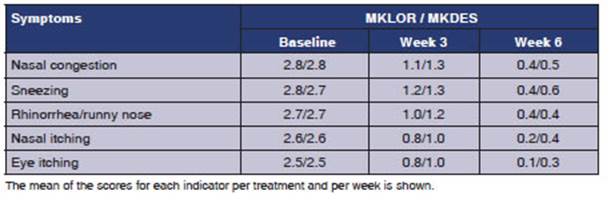
The indicators of the SNOT-20
questionnaire were evaluated by group and by week; both treatments reduced the
scores of the indicators with no differences between the indicators of symptoms
or quality of life (Table 4). The T5SS indicators evaluated
by group and by week showed that both treatments reduced the score, with no
differences between groups (Table 4).
The severity levels
of SNOT-20 indicators in baseline conditions classified about 75% of patients
in the following levels: 4 severe or 5 cant be worse for both groups. At
the sixth week of treatment, more than 90% (91.9% MKLOR and 91.7% MKDES) of
patients were classified as 1 very mild or 0 no problem. The severity
levels of T5SS indicators in baseline conditions classified more than 70% of
patients in maximum level, 3 severe for both groups. At the sixth week of
treatment, more than 90% (91.9% MKLOR and 91.7% MKDES) of patients were
classified in the following levels: 1 mild or 0 none, indicating that both
treatments globally improved the five symptoms under evaluation (Table 5).
The use of the rescue
drug occurred only in 5 of the 86 intention-to-treat patients. Four patients of
the MKDES group were recruited in the same center. 2 of the 5 patients used the
rescue drug in the third week; 3 patients used it during the sixth week; and
the amount of time they used said drug varied from 1 to 18 days. The small
number of patients who used rescue medication does not allow us to infer
whether its use and duration were related to the result of the variables in the
different treatment groups.
Adverse events (AEs)
occurred in 4 of the 86 patients. A total of 12 AEs were reported. Three
patients from the MKLOR group reported 8 AEs and one patient from the MKDES
group reported 4 AEs. One patient showed elevated aminotransferases (> 2
times the reference value), without concomitant medication, and the
investigator attributed it to the MKLOR drug. The event was solved after the
subject suspended treatment and showed an improvement. None of the treatment
groups showed serious adverse events.
DISCUSSION
For some time, leukotriene
receptor antagonists (LRAs) were considered secondary treatment for PAR in
patients with asthma. The information available for PAR without asthma in the
2010 ARIA review showed LRAs with a small benefit in preschool children,
limited efficacy in adults, and a high cost, therefore the recommendations
pointed towards oral antihistamines with a clinical value that was higher than
LRAs.20
A large number of patients with AR dont go to the medical
consultation because they believe that their symptoms are normal; others use
over-the-counter medication, and only a small part go to consultation where
they are diagnosed with moderate or severe PAR.21 In the present study, the
profile of selected patients had a minimum of 3 points in the SNOT-20 score.
These patients could benefit from a combination with the suitable power and
sustained action.
The combined use of
antihistamines and antileukotrienes has been reported to have advantages in
terms of efficacy over monotherapy in patients with PAR. For example, the
combination of montelukast and desloratadine or levocetirizine decreased nasal
symptoms and the levels of eosinophil cationic protein above what had been
observed for the drugs alone.22 The advantage of the
therapeutic combination in terms of health-related quality of life and the
nocturnal symptoms scale, obtained from the Rhinoconjunctivitis Quality
of Life Questionnaire (RQLQ) has also been verified; in addition, the presence
of adverse events was similar for placebo, montelukast, levocetirizine or the
combination of montelukast and antihistamines.23
In the present study,
treatment with montelukast plus loratadine or desloratadine achieved a
difference of more than 3 global points in the SNOT-20 questionnaire in
patients with PAR on week six; this change is clinically relevant and shows the
therapeutic utility of the combination. The 0.8 delta in the SNOT-20 score is
considered clinically significant,16,19 thus, the difference in means
reported here with a confidence interval within a margin lower than said
cut-off point allows us to affirm that the MKDES study treatment is not
inferior to the MKLOR active comparator. The follow-up time used in this study
was comparable to previous studies evaluating the clinical effects of
treatment with montelukast and antihistamines, 22 though shorter than others;23 however, the design of the
present study and the SNOT-20 and T5SS instruments have demonstrated clinically
relevant changes with adequate coverage of the proposed objective, both in the
per protocol population and in the intention-to-treat population. The
evaluation time of six weeks of treatment and total evaluation are justified
according to the criteria of other authors;24 furthermore, the evaluation
period is appropriate for the SNOT-20 primary efficacy instrument in accordance
with the validation history,16, 19 as well as the TSQM
questionnaire. Reported TSQM scores of 84% to 100% are indicative of high
patient satisfaction and excellent tolerability to the combined treatment.
A limitation of this
study is that the evaluation window does not allow checking how symptoms behave
with a long-term treatment, for example, the XPERT study for PAR treated with
levocetirizine evaluated nasal and ocular symptoms with T5SSfrom 4 weeks to 6
months of treatment in order to report the moment in which the symptoms started
to improve and which remained stable throughout the whole treatment. In this
study, levocetirizine improved nasal congestion significantly after the first
month of treatment and continued that way for more than 6 months.18
The evaluation window here was sufficient to verify that the symptoms improved from week 3
with indicators in the mean score of 1 mild, and in week 6 with the mean
score close to 0 none. The evaluation of Ciebiada et al for 32 weeks
demonstrated the long-term effect of montelukast in combination with
desloratadine or levocetirizine. However, their evaluation used a different
instrument focused on nocturnal symptoms.23
Future studies could evaluate the long-term effect of the
combination of antileukotriene plus antihistamine in PAR in the Mexican
population.
The severity levels of the
indicators in the SNOT-20 and T5SS questionnaires showed that quality of life
conditions and symptoms had very high scores in the baseline evaluation: 7 to 8
of every 10 patients were in the highest levels of severity; and six weeks
after the beginning of the study, the scores decreased to a minimum in 9 of
every 10 patients; this improvement was achieved in both treatment groups.
Expected AEs observed in patients
receiving the treatments under evaluation were: cephalea, dyspepsia and
gastrointestinal discomfort, related to the use of montelukast or
desloratadine.24,25 Loratadine doesnt seem
to be related to these symptoms; however, we observed these AEs in both
treatment groups with clinical characteristics that did not show a clear
causal relationship. One patient from the MKDES group presented AEs described
as gastritis, colitis and diarrhea, whereas a patient from the MKLOR
group had acute gastritis. In both cases the causality was reported as
unclassifiable. Cephalea occurred in a patient from the MKLOR group, with
causality reported as conditional due to concomitant consumption of alcohol,
even though it was prohibited. In most cases, concomitant medications were used
to resolve the manifestations. As reported in the literature, the combination
of the drugs under evaluation was safe, considering that no serious was
reported during the study; all reported AEs were milder moderate, only one of
them was from the MKDES group, and one drug-related AE in the MKLOR group was
self-limiting at the end of treatment. With that being said, it is possible to
conclude that the montelukast/desloratadine study medication had adequate
tolerability.
CONCLUSIONS
The combination of desloratadine
and montelukast significantly improves symptoms in patients diagnosed with
PAR. Treatment evaluation showed clinically relevant efficacy and safety that
werent inferior to the combination of montelukast and loratadine. These
results suggest that the oral combination of montelukast + desloratadine 10
mg/5 mg is a good treatment option for adult patients who require a drug with
an action mechanism different from that of antihistamines for controlling the
signs and symptoms of the disease.
Conflict of interest
Livan Delgado-Roche is an
employee of Laboratorios Liomont, S.A. de C.V. The rest of the authors have no
conflict of interest to declare.
Acknowledgement
The authors want to thank the
Infinite Clinical Research International CRO (Clinical Research Company
Contract) for its support in the management and monitoring of the clinical
trial.
REFERENCES
1. Ciprandi G, Natoli V, Puccinelli P, et al. Allergic rhinitis: the eligible candidate to mite immunotherapy in the
real world. Allergy Asthma Clin Immunol 2017;13:11.
https://doi.org/10.1186/s13223-017-0185-x
2. Mancilla-Hernandez E, Medina-Avalos MA, Barnica-
Alvarado RH, et al. Prevalencia de rinitis alérgica en poblaciones de
varios estados de México. Rev Alerg Mex 2015;62:196-201.
https://doi.org/10.29262/ram.v62i3.107
3. Instituto Mexicano del Seguro Social. Guía de
Práctica Clínica. Diagnóstico y Tratamiento de la Rinitis
Alérgica. Actualización 2017. Disponible en:
http://www.imss.gob.mx/profesionales-salud/gpc. Consultado: 28-Jun-2022.
4. Brozek JL,
Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA)
guidelines-2016 revision. J Allergy Clin
Immunol 2017;140:950-8.
https://doi.org/10.1016/j.jaci.2017.03.050
5. SIIC. Rinitis alérgica. In: Sociedad Iberoamericana
de Información Científica, editor. Guías Distinguidas.
Enfermedades Respiratorias. Vol 1 (No 1). Buenos Aires: SIIC; 2012. p. 3-26.
6. Negro Álvarez JM, Rodríguez Pacheco R.
Rinitis alérgica. In: AEM, editor. Actualizaciones El Médico.
España: AEM; 2011. p. 1-30.
7. Bousquet J, Schünemann
HJ, Togias A, et al., Next-generation Allergic Rhinitis and Its Impact on
Asthma (ARIA) guidelines for allergic rhinitis based on Grading of
Recommendations Assessment, Development and Evaluation (GRADE) and real-world
evidence. J Allergy Clin Immunol. 2020;145:70-80.e3.
8. Aristizabal MS, Martínez FM, Ropero J, et al.
Rinitis alérgica en el mundo moderno. S&EMJ. 2021;2:5-17.
9. Schering-Plough S.A. de C.V.
Montaclar. Información para prescribir amplia [Internet]
2016. Disponible en:
https://com-epublishmerck-content.s3.amazonaws.com/tridion-deployer/us-live-epublish/profesionales.msd.com.
mx/secure/pdf/MONTACLAR%20IPP.pdf.
10. Velázquez de Campos O. Combinación
montelukast-desloratadina en las enfermedades alérgicas en los
niños. Arch Venez Farmacol Ter 2013;32:34-8.
11. Gorena Antezana S, Imaña C, Mendoza Amatller
A. Fármacos antitusivos y antihistamínicos. Rev Bol Ped
2005;44(2).
12. Church MK, Church DS. Pharmacology of antihistamines. Indian journal of
dermatology 2013;58:219-24.
https://doi.org/10.4103/0019-5154.110832
13. Anthes JC, Gilchrest H,
Richard C, et al. Biochemical characterization of desloratadine, a potent
antagonist of the human histamine H(1) receptor. Eur J Pharmacol 2002;449:229-37.
https://doi.org/10.1016/S0014-2999(02)02049-6
14. Cingi C, Oghan F, Eskiizmir G, et al. Desloratadine-montelukast combination improves quality of life and
decreases nasal obstruction in patients with perennial allergic rhinitis.
International forum of allergy & rhinology 2013;3:801-6.
https://doi.org/10.1002/alr.21185
15. Wilson A. Antihistamines
alone and in combination with leukotriene antagonists in nasal congestion. Clin Exp Allergy Rev 2002;2(3):95-100. https://doi.org/10.1046/j.1472-9725.2002.00045.x
16. Breinbauer H, Varela C, Núñez M, et al.
Encuesta de síntomas SNOT-20 para rinitis alérgica y
rinosinusitis: validación en Chile. Rev Med Chile 2011;139:886-95.
https://doi.org/10.4067/S0034-98872011000700009
17. Rogkakou A, Villa E, Garelli
V, et al. Persistent Allergic Rhinitis and the XPERT Study. The World Allergy
Organization journal 2011;4(3 Suppl):S32-6.
https://doi.org/10.1097/1939-4551-4-S3-S32
18. Atkinson MJ, Sinha A, Hass
SL, et al. Validation of a general measure of treatment satisfaction, the
Treatment Satisfaction Questionnaire for Medication (TSQM), using a national
panel study of chronic disease. Health Qual Life Outcomes 2004;2:12. https://doi.org/10.1186/1477-7525-2-12
19. Piccirillo JF, Merritt MG,
Jr., Richards ML. Psychometric and clinimetric validity of the 20-Item Sino-
Nasal Outcome Test (SNOT-20). Otolaryngol Head Neck
Surg 2002;126(1):41-7. https://doi.org/10.1067/mhn.2002.121022
20. Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010
revision. J Allergy Clin Immunol 2010;126:466-76.
https://doi.org/10.1016/j.jaci.2010.06.047
21. Bousquet J, Hellings PW,
Agache I, et al. ARIA 2016: Care pathways implementing
emerging technologies for predictive medicine in rhinitis and asthma across
the life cycle. Clin Transl Allergy 2016;6:47.
https://doi.org/10.1186/s13601-016-0137-4
22. Ciebiada M, Gorska-Ciebiada M, DuBuske LM, et al. Montelukast with desloratadine or levocetirizine for
the treatment of persistent allergic rhinitis. Ann Allergy Asthma Immunol 2006;97:664-71.
https://doi.org/10.1016/S1081-1206(10)61098-8
23. Ciebiada M, Ciebiada MG, Kmiecik T, et al. Quality of life in patients with persistent allergic rhinitis treated
with montelukast alone or in combination with levocetirizine or desloratadine.
J Investig Allergol Clin Immunol 2008;18:343-9.
24. Villar López J, Lizán Tudela L, Soto
Álvarez J, et al. La satisfacción con el tratamiento. Aten
Primaria 2009;41:637- 45.
https://doi.org/10.1016/j.aprim.2008.10.021
25. European Medicines Agency. Aerius. Informe
Público Europeo de Evaluación (EPAR) EMA/376282/2015 Spanish
[Internet] 2015. Disponible en:
https://www.ema.europa.eu/en/medicines/human/EPAR/aerius.