Autor : Tomicic, Vinko1-2, Catalioti, Frank1, Mendoza, Sheyla3
1Coronary Care Unit. Hospital Regional Dr. Leonardo Guzmán, Antofagasta, Chile. 2Faculty of Medicine and Dentistry, Universidad de Antofagasta. 3Instituto Regional de Enfermedades Neoplásicas Norte, Trujillo, Perú.
https://doi.org./ramr.10.56538/GHKS7463
Correspondencia : Vinko Tomicic E-mail: vtomicic@gmail.com
ABSTRACT
A 20-year-old male with known
asthma diagnosis arrived at the Emergency DepartÂment of a hospital in his town
with history of dyspnea 1 day before admission. The paÂtient then became tachycardic, tachypneic and
cyanotic and received emergency intuÂbation. At the ICU (Intensive care Unit)
of the tertiary care general hospital, he showed severe bronchospasm, high
airway pressure during mechanical ventilation (MV) and severe hypoperfusion. He received crystalloids and norepinephrine
for resuscitation. On the third day, he developed subcutaneous emphysema,
pneumothorax and hyperÂcapnia with mixed acidosis. We
decided to use ultra-protective mechanical ventilaÂtion concomitant with Novalung®.
With this strategy, we were able to reduce airway pressures, iPEEP (intrinsic positive end-expiratory pressure) and
resistive mechanical power (MP) and improve hypercapnia
and acidosis. The patient was connected to Novalung® for ten days
and showed good evolution. Finally, he was extubated
and discharged from the ICU, and left the hospital in good condition.
Key words: Status asthmaticus, Ventilator-induced lung
injury, Extracorporeal circulation, Barotrauma
RESUMEN
Paciente varón de 20 años, con
diagnóstico de asma conocida, llegó al departamento de
emergencias de un hospital de su localidad con historia de disnea 1 d antes de
la admisión. Posteriormente, se torna taquicárdico,
taquipneico y cianótico, por lo que fue
intubado de emergencia. En la UCI del hospital general de tercer nivel,
presentó bronÂcoespasmo grave, presiones de vía aérea
elevadas durante la ventilación mecánica e hipoperfusión
grave. Recibió cristaloides y norepinefrina como resucitación. Al
tercer día, presentó enfisema subcutáneo,
neumotórax e hipercapnia con acidosis mixta. Se decidió utilizar
ventilación mecánica ultraprotectora
asociada con Novalung®. Con esta estrategia, logramos reducir
las presiones de la vía aérea, la PEEPi,
la potencia mecánica (PM) resistiva y mejorar la hipercapnia y la
acidosis. El paciente permaneció 10 d en Novalung® y
mostró buena evolución posterior. Finalmente, es extubado, dado de alta de la UCI y salió del
hospital en buenas condiciones.
Palabras clave: Estado asmático, Lesión pulmonar inducida por
ventilación mecánica, CircuÂlación extracorpórea, Barotrauma
Received: 05/23/2022
Accepted: 09/01/2022
INTRODUCTION
It is known that mechanical
ventilation (MV) produces per se injuries in the pulmonary fibrous
skeleton.1 This damage is associated with
lung compliance resistance, and the adjustment of tidal volume (TV), inspiratory
flow, PEEP level and respiratory rate (RR); the latter being related to the
amount of times the lung is subjected to an abnormal breathing pattern per unit
of time,2
generating an inflammatory process with positive feedback
(ventilator-induced lung injury vortex [VILI Vortex]).3,
4
The status asthmaticus
(SA) is developed with high airway pressures, where the resistance eleÂment is
the most important one. Even though the driving pressure (DP) is not a problem,
baroÂtrauma is also developed. In order to control the consequences of the
reduction in T V and RR (hypercapnia and respiratory acidosis), an extraÂcorporeal
CO2 remover is added (ECCO2R), which achieves
decarboxylation using low blood flow and low sweep flow.5-7
The mechanical power (MP) is
divided into its three components, and the magnitude of the “resistive power”
is described as being the one reÂsponsible for the MP. The reduction of the RR
and TV interrupts the dynamic hyperinflation cycle, thus reducing intrathoracic pressure, correcting acidosis, allowing for
the attenuation of hypoxic pulmonary vasoconstriction (HPV) and reducing the postload of the right ventricle.
We present the case of an
asthmatic patient with life-threatening risk who evolved with refractory hypercapnia, mixed acidosis with blood hypertenÂsion and
barotrauma, and was treated with arteÂriovenous ECCO2R (Novalung®).
CASE REPORT
20-year-old male with known
asthma who arrived at the Emergency Service (ES) of the regional hosÂpital of
Antofagasta; he had been referred from ToÂcopilla. He
complained of breathing difficulty one day before admission. Then he showed
tachypnea (30 rpm), with circumoral cyanosis and
respiratory muscle fatigue. Due to this condition, he received orotracheal intubation and was subjected to MV deeply
sedated and with neuromuscular blockade. He received norepinephrine due to the
hemodyÂnamic compromise.
He was admitted to the emergency
service with an APACHE II score of 11 points. MV was first delivered via
volume-controlled mode with a TV of 350 mL, RR 24, I:E
ratio = 1:3, PEEP 3 cmH2O
(intrinsic PEEP = 18 cmH2O)
and FiO2 of 50%. The patient
showed high inspiratory pressure (90 cmH2 O),
so nebulization with salbutamol and Berodual®
(fenoterol 0.25mg/mL + ipratropium
bromide 0.5 mg/mL) was intensified. The ausÂcultation revealed a bilateral
decrease in breath sounds, with generalized wheezing. Respiratory monitoring
showed a plateau pressure (Pplat) of 17 cmH2O and a static
compliance of 30 mL/ cmH2O.
Initial arterial blood gases: pH = 7.18, PCO2
= 50.5 mm/Hg, PaO2 = 89.3 mmHg, HCO3 = 18.2 mEq/L.
Negative PCR test for COVID-19.
At the ICU, the patient continued
with severe bronchospasm, desaturation up to 63% with inÂcreasing doses of
noradrenaline (from 0.06 μg/kg/ min to 0.2 μg/kg/min) and hypothermia tendency. Control tests showed lactic acidosis
(10.2 mMol/L) with pH of
7.17 and HCO3 of 18 mEq/L. On the third day, the subject had palpable cervical
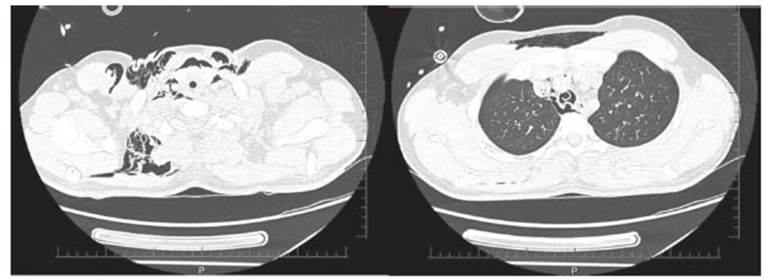
crackÂling sounds, and the chest scan showed cervical subcutaneous emphysema
and pneumothorax (Figure 1). Thus, a pleural tube was placed and the ventilatory schedule was modified. Methylprednisolone
boluses were included (500 mg x three times). The TV was reduced to 3.4 mL/kg
of predicted body weight, RR was reduced to 10 rpm, inspiratory time to 0.72 s,
minute ventilation (VE) to
2.6 L/min and the I:E ratio to 1:7, without PEEP. With
this pattern, the Pmax decreased to 48 cmH2O and the iPEEP reached 6 cmH2 O.
Due to the hypercapnia, it was associated with arteÂriovenous ECCO2R
(Novalung®).
Aminophylline was included. No fever or evidence of septic focus detected.
Once the patient was connected to
Novalung®,
we applied the volume-controlled mode with a TV of 300 ml and a RR of 10 rpm.
The Novalung® blood flow was maintained between
1.2 L/min and 1.6 L/min, and the sweep flow was adjusted between 6 L/min and 7
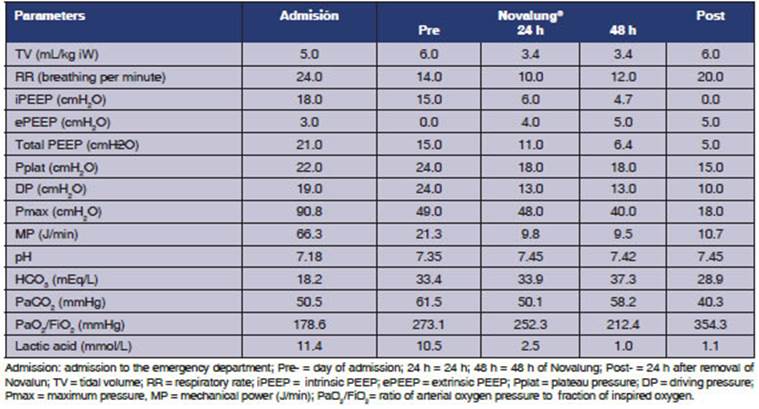
L/min. Table 1 shows the PaO2/FiO2 ratio, the PaCO2 and pH on the day the Novalung® device was connected.
Twenty-four hours after being connected to Novalung®, the maximum pressure (Pmax) of the airways was reduced and oxygenation remained
unaffected. The most important modifications were a drop in the iPEEP and Pmax (Figure 1).
The patient remained connected to
Novalung® for ten days and showed good
evolution. 72 h after removing the Novalung® device, the patient was extubated. Ventilatory parameters
before extubaÂtion: Pplat =
15 cmH2O; DP = 10 cmH2O; Pmax
= 18 cmH2O and MP of 10
J/min, without iPEEP. Gasometric
parameters: pH = 7.44, PaCO2 =
39.6 mmHg, PaO2 =
71.9 mmHg. Give the patient’s stability, he was
discharged from the ICU 120 h after extubation,
having solved the bronchospasm.
DISCUSSION
The most important finding was to
identify the resistive mechanical power as the leading cause of barotrauma in a
patient with SA. A significant correlation was observed between the iPEEP and Pmax of the airway.
Both decreased drastically when we were able to reduce the RR and TV and extend
the expiratory time (I:E ratio = 1:7) after installing
the Novalung® device.
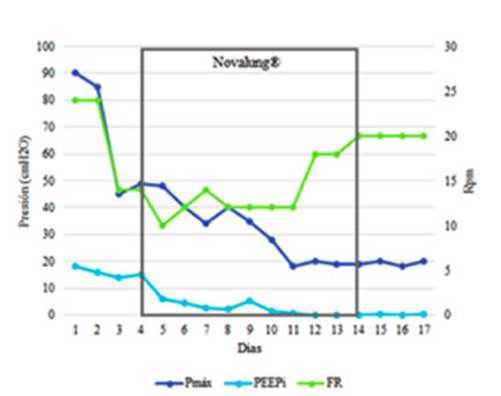
After introducing
this device, the RR could be reduced from 24 rpm to 10 rpm. Thus, the iPEEP was reduced from 15 cmH2 O
to 6 cmH2O. This change
reduced pulmonary hyperinflation and probably reduced the intrathoracic
pressure, imÂproving venous return and cardiac output; this was reflected in
the improvement of clinical perfusion, diuresis, and the correction of lactic
acid. Acidosis control should reduce the HPV and postload
of the right ventricle8 (Table 1).
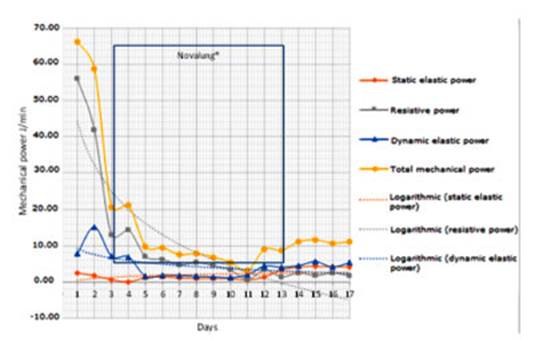
When we analyzed the
specific components of the MP, such as the static elastic power (associated
with PEEP), the dynamic elastic power (associÂated with TV) and the “resistive
power” (native airway), we observed that the drop in the MP was caused mainly
by the reduction of the resistive component, theoretically the most important
one in status asthmaticus. In our case, this
component reached more than 80% of the total MP on the first day (Figure 2).
When the exhalation
of gas is incomplete beÂcause the next inspiration begins before the lung is
completely empty, air trapping occurs and the expiratory time constant (τ) may
reach values near 0.9 s.9 For that reason, the expiratory time must be extended. The
reduction of the TV and RR also reduced the minute ventilation (VE), which is the main cause of
dynamic hyperinflation.9 Twenty-four hours after the
installation of the Novalung® device,
we observed the impact that the reduction in the RR and the TV had on the
“resistive power”, which decreased from 58 J/min to 14.6 J/min.
These patients often
show increased respiraÂtory effort and are dehydrated, and develop lactic
acidosis, worsening respiratory acidosis. All these elements were present in
our patient on admission, so he received crystalloids, norepinephrine and low
levels of PEEP (Table 1).
The MP considers all
the elements to be inÂcluded in the Otis equation. The DP and RR are the most
aggressive for the pulmonary fibrous skeleton.10 On the other hand, the peak flow is also an important variable for the
development of alveolar epithelial damage (Figure 3). ThereÂfore, the decrease
in the inspiratory flow avoids the disruption of the respiratory epithelium.
This phenomenon has been shown in an in vitro model of Tschumperlin.11
In our patient, as a consequence of the reduction in the RR, the
expiratory and inÂspiratory time could be simultaneously extended and so the
peak flow could be reduced.
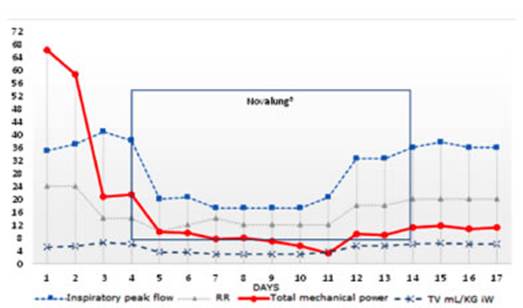
The impact of the MP
has been studied in relaÂtion to the acute respiratory distress syndrome
(ARDS), but there aren’t enough studies that reÂlate it to asthmatic
decompensation.12-14 However, regardless of the
specific damage patterns, the dynamic elastic power (ARDS) or resistive power
(status asthmaticus), the mechanical power is inevitably transferred to the pulmonary fibrous skeleton in each
mechanical cycle.
The resistive component of the MP
must always be analyzed in patients with airway obstruction. When analyzing the
components separately, the resistive component (grey line) clearly stands out
as the main MP generator in this type of patients (Figure 2).
CONCLUSION
In short, when patients evolve
with high airway pressures, despite the existence of a suitable plateau
pressure, we must consider the resistive component of the MP as the origin of
barotrauma (bronchial obstruction). Through simple formulas we can predict the
impact of mechanical ventilator variables on asthmatic patients.10
The ECCO2R
systems are a safe tool to be associated with ultra-protective MV in severe
asthmatic patients.
Conflict of interest
The authors declare that there is
no conflict of interest.
REFERENCES
1. Ranieri VM, Suter PM, Tortorella C et al. Effect of mechanical ventilation on inflammatory mediators in patients
with acute respiratory distress syndrome: a ranÂdomized controlled trial. JAMA
1999;282:54-61. https://doi.org/10.1001/jama.282.1.54
2. Marini JJ, Rocco PR. Which
component of mechanical power is most important in causing VILI? Crit Care 2020;24:39.
https://doi.org/10.1186/s13054-020-2747-4
3. Marini JJ. How I optimize power
to avoid VILI. Crit Care 2019;23:326.
https://doi.org/10.1186/s13054-019-2638-8
4. Marini JJ, Gattinoni
L. Time Course of Evolving VentiÂlator-Induced Lung Injury: The “Shrinking Baby
Lung”. Crit Care Med. 2020;48:1203-9.
https://doi.org/10.1097/CCM.0000000000004416
5. Kukita
I, Okamoto K, Sato, T et al. Emergency extraÂcorporeal life support for
patients with near-fatal status asthmaticus. Am J Emerg Med 1997;15:566-9.
https://doi.org/10.1016/S0735-6757(97)90158-3
6. Lobaz
S, Carey M. Rescue of acute refractory hyperÂcapnia
and acidosis secondary to life-threatening asthÂma with extracorporeal carbon
dioxide removal (ECÂCO2R). J Intens Care Soc 2011;12:140-2.
https://doi.org/10.1177/175114371101200210
7. Augy
JL, Aissaoui N, Richards C
et al. Two years multiÂcenter multicenter
observational prospective, chort study on
extracorporeal CO2 removal
in a large metropolis area. J Intens Care Soc 2019;7:45.
https://doi.org/10.1186/s40560-019-0399-8
8. Peinado
VI, Santos S, Ramirez J, Rodriguez-Roisin R and Barberá JA. Response to hypoxia of pulmonary
arteries in chronic obstructive pulmonary disease: an in vitro study. Eur Respir J. 2002;20:332. https://doi.org/10.1183/09031936.02.00282002
9. Tuxen
DV, Lane S. The effects of ventilatory
pattern on hyperinflation, airway pressures, and circulation in mechanical
ventilation of patients with severe air-flow obstruction. Am Rev Respir Dis 1987;136:872-9.
https://doi.org/10.1164/ajrccm/136.4.872
10. Costa ELV, Slutsky A, Brochard LJ, et al. Ventilatory Variables and Mechanical Power in Patients with
Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2021;204:303-11.
https://doi.org/10.1164/rccm.202009-3467OC
11. Tschumperlin
DJ, Margulies SS. Equibiaxial deformation-induced
injury of alveolar epithelial cells in vitro. Am J Physiol. 1998; 275:L1173-83.
https://doi.org/10.1152/ajplung.1998.275.6.L1173
12. Scharffenberg
M, Wittenstein J, Ran X, et al. Mechanical Power
Correlates with Lung Inflammation Assessed by Positron-Emission Tomography in
Experimental Acute Lung Injury in Pigs. Front Physiol. 2021;12:717266.
https://doi.org/10.3389/fphys.2021.717266
13. Demoule
A, Brochard L, Dres M et
al. How to ventilate obstructive and asthmatic patients.
Intens Care Med 2020;46:2436-49.
https://doi.org/10.1007/s00134-020-06291-0
14. Marini JJ (2011) Dynamic
hyperinflation and auto-positive end-expiratory pressure: lessons learned over
30 years. Am J Respir Crit Care Med 184:756–62.
https://doi.org/10.1164/rccm.201102-0226PP