Autor : Gasteneguy Rodrigo1, González Claudio2, Saadia Otero Marcela3, Fernández Florencia4, TurĂłn Gonzalo5, Castro Ignacio6, Larrateguy Santiago7, Armelino Javier8, Miguel Mauricio9, Alvarez Marcelo10, COLLABORATOR Cigarra Cecilia1, Lebus Janina2, OlguĂn Emilia3, Conti Ernesto4, Cuello Juan Ignacio5
AUTHORS 1Hospital Municipal de Coronel Suárez “Dr. RaĂşl A. Caccavo”. Coronel Suárez. Buenos Aires. 2 Hospital General de Agudos JosĂ© M. Ramos MejĂa. CABA 3Hospital de RehabilitaciĂłn Respiratoria MarĂa Ferrer. CABA. 4Hospital General de Agudos Enrique TornĂş. CABA 5Hospital Italiano de Buenos Aires. CABA. 6Hospital Bouquet Roldan. NeuquĂ©n. 7Centro Privado de Medicina Respiratoria. Universidad Adventista del Plata. Paraná. Entre RĂos. 8Hospital de ClĂnicas JosĂ© de San MartĂn. 9 Sanatorio Británico. Centro de KinesiologĂa CrĂtica. Rosario. Santa Fe. 10Hospital Zonal General de Agudos Julio de Vedia. 9 de Julio. Buenos Aires. COLLABORATORS 1Hospital Interzonal General de Agudos Petrona Villegas de Cordero. San Fernando. Buenos Aires 2Consultorio NeumokinĂ©sico Avellaneda. Santa FĂ© 3Hospital Italiano de San justo. Buenos Aires 4Instituto Cordis. Resistencia. Chaco. 5Hospital Municipal “Eva PerĂłn” de Coronel Dorrego. Buenos Aires.
https://doi.org/10.56538/ramr.KVKV4268
Correspondencia : Dr. Rodrigo Gasteneguy. E-mail: mgasteneguy@gmail.com
Received: 09/16/2021
Aceptado: 03/27/2022
INTRODUCTION RATIONALE AND JUSTIFICATION OF THE DOCUMENT
In December 2019, the first case
of the disease caused by the SARS-Cov-2 virus was detected in the city of
Wuhan, China.1 Unlike the
limited character of the two previous epidemics, the Middle East Respiratory
Syndrome (MERS) and the Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV), the rapid expansion of SARS-CoV-2 forced the World
Health Organization (WHO) to declare the pandemic in March, 2020.2
According to the reports on the
evolution of the pandemic available at the John Hopkins University (JHU), at
the time this document was written, around 160 million cases and 3.3 million
deaths had been reported.3 During the
first year, the pandemic caused 1.8 million deaths around the world, compared
to 2.6 million deaths produced by all the lower respiratory tract infections in
2019.4
The mortality produced by all the
respiratory inÂfections in 2017 (last available record) was 64,869 deaths, and
the mortality produced by SARS-CoV-2 only within one year of the pandemic
accounted for 53,741 deaths.5,6
Apart from the mortality produced
by SARS-CoV-2, we must consider two other levels of impact: the first one,
generated by the acute disease, reÂquires the early intervention of the
rehabilitation tool, such as in the ICU (Intensive Care Unit) and in-patient
wards. The second level of impact refers to the chronic disease, the multiple
physical, psyÂchological and neurocognitive functional sequelae
that are usually expressed as Post-Intensive Care Syndrome (PICS) in critically
ill patients.
A national study conducted on a
sample of 207,000 patients with complete data, treated beÂtween March and
October 2020, allows us to have an approximate estimation of how many patients
who suffered from the disease caused by SARS-CoV-2 required rehabilitation.7 20.1% of them
(41,703 patients) were hospitalized, out of which 2.7% (5,652 patients) were
admitted to the ICU. Only among these ICU survivors (around 2,800 patients),
then in the intermediate care ward, and finally with the outpatient in-person
or remote modality, would rehabilitation be justified in that context.
Apart from the patients admitted
to the ICU, the indication should also include patients with modÂerate or
severe forms of the disease who required different levels of oxygen therapy in
intermediate care or general wards.
In view of the above, an early
intervention is urgently needed, mainly in the respiratory, cardioÂvascular,
neuromotor, cognitive and psychological areas, in
order to minimize sequelae and try to reach maximum
patient’s autonomy and the best possible quality of life.8
In any case, the most important
thing is that rehabilitation becomes a continuous intervention. We
recommend to keep a common line of work throughout the
different stages of disease evoluÂtion. This applies to both patients who begin
this intervention in the ICU, continue in the general ward and then during the
outpatient period, and to those who begin in the general ward and continue on
an outpatient basis.
The objective of this
document is to offer the professionals involved in the respiratory rehabiliÂtation
of these patients a set of recommendations supported by the current state of
knowledge and endorsed by our specialized experts that can be feasibly used in
centers of different complexity levels in our country.
CLINICAL PRESENTATION OF INFECTION BY SARS-CoV-2
Infection by SARS-CoV-2 can be
symptomatic or asymptomatic. Symptomatic patients may show mild and moderate or
even severe forms of the disÂease with pneumonia and ARDS (acute respiratory
distress syndrome), with respiratory failure and multi-organ failure. Also,
long-term complications could occur after SARS-CoV-2 infection, causing the
post-COVID syndrome (pCS) or the persistent COVID
syndrome (PC).
Around 80% of patients with
COVID-19 develop mild to moderate disease; 15% progress to severe stages and
require oxygen support, and 5% develop a critical disease including ARDS,
septic shock and multi-organ failure.9
Age and various comorbidities such as diabetes, obesity, lung and
cardiovascular diseases and some genetic polymorphisms corÂrelate with a higher
risk of respiratory failure.10-12
We must also take into account
that approxiÂmately 50% of people with severe pneumonia caused by COVID-19
develop ARDS, with pulmoÂnary fibrosis as a common complication.13
These patients will have damaged lung
function with irreversible respiratory failure associated with bad prognosis.14
A. RESPIRATORY REHABILITATION IN COVID-19 PATIENTS ADMITTED TO THE
CRITICAL CARE UNITS
Patients with COVID-19 whose treatment
requires hospitalization in the Intensive Care Unit (ICU) with or without
invasive mechanical ventilation (IMV) need early kinesiology care, not only for
the management of the ventilatory treatment, but also
for the motor rehabilitation that is necessary for the patient to go back to
his/her regular activities after discharge.
In this section we suggest that
general guideÂlines are established regarding the way in which we should
evaluate the impact of rehabilitation upon these patients, which tests can be
done, and how to address the rehabilitation process of COVID-19 patients in the
ICU.
The first thing to decide is how
the rehabilitation plan should be organized, taking into account that it has to
be individualized and customized. In order to do that, several aspects are to
be considered:
1. Setting suitable titration of
analgesia and sedaÂtion, depending on the ventilatory
mode that is being used, disease evolution, and the patient’s oxygenation
state.
2. Using a ventilator mode and
setting that are adequate for the patient (avoid the patient/ ventilator
asynchrony), on distension and hyÂpoventilation.15
3. Providing the kinesiology
treatment gradually, taking into account the clinical status of the patient.
4. Monitoring with strict safety criteria.16
5. Planning early rehabilitation
together with the interdisciplinary team.
When addressing the important
aspects deÂscribed in this section, we use four trigger quesÂtions for
educational purposes.
1. Which are the objectives of a
rehabilitation process in the ICU?
The main objective of an early
rehabilitation proÂgram (ER) (defining the ER as an intervention to provide
motor, sensitive and proprioceptive stimuli that generate in the patient a less
negative impact of the ICU admission), is to avoid losing the funcÂtionality
the patient had before being admitted to the critical care area.17
Also, the objectives related to
the ER must be proposed, for example, reducing sedation and anÂalgesia, maintaining
the range of motion, sitting position, standing position and walking. Then come the DLAs (daily living activities).
These goals have to be proposed
upon the paÂtient’s admission to the critical care area, and must be evaluated
upon discharge.
For the correct organization of
the proposed objectives, the measures known as the “ABCDEF Bundle” can be used,
especially when there is early weaning, prevention and treatment of delirium
and early rehabilitation.18 This allows
for the coÂordination of patient care in order to wean him/ her from IMV and
discharge him/her from the ICU.
2. Which are the necessary
criteria to begin rehabilitation?
The kinesiologist
has to adapt to the patient’s conÂditions: whether he/she has orotracheal intubation or tracheotomy, invasive or
non-invasive mechaniÂcal ventilation, humidified high-flow therapy or any other
form of oxygen therapy support. It is essential to consider the presence of
drug adminÂistration routes, drainage, hemodynamic stability and monitoring.
The patient must have a stable
medical condiÂtion, one airway free of complications and ensured oxygen
requirement, and he/she should also begin the respiratory rehabilitation (RR)
session, ensurÂing the use of drugs if necessary.
The criteria are defined in the
following way:19
1. Heart rate of less than 50% of
the theoretical maximum heart rate (TMHR).
2. Blood pressure with a
variability of less than 20% (avoid hemodynamic decompensation).
3. Normal electrocardiogram.
4. Partial oxygen saturation >
90% with a reducÂtion of less than 4 points at the time of the ER.
5. PaO2/FiO2
> 300 (ER tolerance index with good reserve volume; lower
values reduce such volÂume, state of alert).
6. Adapted respiratory pattern.
7. Stable mechanical ventilation.
8. Stable airway.
9. Absence of fever.
3. How is the patient who begins
rehabilitation in the ICU evaluated?
The evaluation must include
respiratory and musÂcular functions and state of consciousness. The recommended
instruments are:
1. Evaluation of dyspnea through
the mMRC (Modified Medical Research Council) scale.20
2. Evaluation of the muscular
state through the MRC scale.21
3. Assessment of sedation and
analgesia and paÂtient’s state of alert: Visual Analog Scale (VAS), Pain
Behavior Scale (PBS) Richmond Agitation- Sedation Scale (RASS), and delirium
scale (CAM-ICU, Confusion Assessment Method for the Intensive Care Unit).22-24
4. Which are the elements of the
ICU’s early rehabilitation plan?
Stages must be respected according
to the Morris model of complexity levels25.
The plan consists of the
following steps:
• Including two daily stimuli
from the patient’s admission to the critical care area until disÂcharge.
• The initial level (deeply
sedated patient) inÂcludes passive movement of the limbs and postural control.
• Once the patient regains
consciousness, he/ she begins with active-assisted
exercises and functional progression as he/she meets the objectives. Such
progression includes: sitting on the corner of the bed, trying the standing
position once he/she controls his/her trunk, and then walking around with
assistance and doing activities outside the bed. 25,26
• Including family members in the
rehabilitaÂtion process through videocalls and helping
the patient both with functional progression and providing the patient’s
elements (watch, glasses, books, radio, etc.)
• Recording adverse events so as
to avoid repeatÂing them.
B. RESPIRATORY REHABILITATION ON THE IN-PATIENT WARD
As we already mentioned, it is
estimated that between 14% and 20% of patients infected with SARS-CoV-2 will
require hospitalization in a genÂeral in-patient ward, so complications
associated with immobilization could generate a negative impact on the
patient’s quality of life.7,27 Thus, it is
essential that the patient receives respiratory rehabilitation treatment during
hospitalization, for the prevention and timely management of physical
deconditioning effects and effects related to the appearance of sequelae.28
When the patient is transferred
from the ICU to Intermediate Care or to the in-patient ward, the RR has to be
continuous and in-line with the treatment that had already begun in the ICU; in
the case of patients initially admitted to the in-patient ward, they have to
meet the following conditions once they are included in the rehabiliÂtation
program:
1. Patients coming from the ICU, will continue with their RR treatment but those who are
directly admitted to the in-patient ward have to establish their corresponding
treatment.
2. An evaluation will be carried
out to identify prognostic factors of PICS syndrome, chronic damage caused by
COVID, post-COVID synÂdrome and persistent COVID syndrome, in patients coming
from the ICU.29
3. Rehabilitation goals have to be
set.27,30
4. Patient’s evolution has to be
monitored.
5. The comparison between the RR
parameters and applications in its different stages is recomÂmended.
Three triggering questions are
included in this section that intend to address to whom, how and when
to perform the RR in a general in-patient ward.
1. Which are the conditions for COVID-19 patients to begin RR on the
in-patient ward?
According to the aforementioned,
around 3-5% of moderately ill patients will develop severe or even critical disease
7 to 14 days after the onset of the infection31,32.
The parameters that should be
evaluated in patients coming from the ICU are: 31-33
1. Time since the onset of
symptoms.
2. Type and number of symptoms.
3. Oxygen saturation values.
4. Intensity and extent of
pulmonary involvement.
5. Supplemental oxygen
requirement and types of administration.
6. Need to use invasive or
non-invasive mechanical ventilation.
7. Ventilation time and possible
complications.
8. Coexistence of renal, hematologic,
neurologic or any other type of complication and type of treatment received.
9. In patients directly admitted
to the in-patient ward, an observational behavior must be set, depending on the
patient’s evolution.
A. EXCLUSION AND TERMINATION OF
EXERCISE CRITERIA
A.1 EXCLUSION CRITERIA27,31
– Patient with fever.
– Time of initial consultation ≤
7 days in patients directly admitted to the in-patient ward.
– Duration of the disease ≤
3 days from onset to appearance of dyspnea, due to disease progresÂsion or
fully active clinical condition.
– Progression of opacities in
chest X-ray of at least 50% in 24 to 48 hours.
– SO2 ≤ 90% with supplemental oxygen.
– Heart rate < 40 or > 130 bpm.
– Blood pressure at rest <
90/60 or > 140/90 mmHg.
– Respiratory rate > 24 bpm.
– Lack of consent from the
patient.
A.2 TERMINATION OF EXERCISE
CRITERIA 27,31,33
– Modified Borg Scale value >
3 for dyspnea score at the initial stage of RR.
– Drop in SpO2 > 4%.
– Signs of chest tightness.
– Alterations in ventilatory mechanics and/or use of accessory muscles.
– Breathing difficulty,
dizziness, headache, blurry vision, palpitations, excessive sweating and balÂance
disorder.
– Other conditions determined by the
physician as inadequate for doing the exercise.
2. How should patients included in the rehabilitation intervention be
evaluated?
The different evaluations
described below shall be selected depending on the working context of each
professional.
There are different fields within
the scope of the evaluation:
1. EVALUATION OF THE PATIENT’S
GENERAL CONDITION
It will observe the breathing
rhythm, the state of muscular masses, mobility and range of motion, state of
consciousness and the possibility to cooperÂate in the rehabilitation.
2. EVALUATION OF DYSPNEA
In order to evaluate the level of
dyspnea, many validated, simple scales can be used.
2.1 Modified Borg Scale: to evaluate the level of effort perceived by the patient and to be
able to prescribe and control the intensity of the activity27.
2.2 Visual Analog Scale 34
3. EVALUATION OF EXERCISE
CAPACITY
If allowed by the respiratory,
cardiac and metabolic reserves of the patient, the following tests can be done:
3.1 1- MIN SIT-TO-STAND TEST (STS1’): this test will allow the evaluation of desaturation induced
by exercise.35
3.2 5R-STS: normal cut-off point ≤ 12 secÂonds.36
3.3 TEST TIME UP and Go (TUG): abnormal cut-off point for fall risk shall be ≥ 16 seconds.37
3.4 4 - METRE GAIT SPEED: this test will evaluate the time needed to walk 4 meters at a normal
speed. A value > 0.8 m/sec shall be conÂsidered abnormal.38
4. STRENGTH ASSESSMENT
3.1 Medical Research Council
Scale (MRC)27 .
3.2 Repetition method.39-41
4 EVALUATION
OF DAILY LIFE ACTIVITIES (DLAS)
4.1 PCFS29
4.2 Barthel
Index42
4.3 Katz Index43
3. When and how should these patients undergo the peripheral muscle
training?
We suggest early rehabilitation
in patients comÂing from the ICU and in those who are directly admitted to the
in-patient ward during the first 3 days after the patient was
stabilized. It is also important to have good pain control, in order to favor
the achievement of objectives.27
The design of RR programs for
patients with COVID-19 must respect the general principles of training, which
are related to intensity, duration, frequency, specificity and exercise
reversibility.30,44
To do that, the training
objectives and scope have to be planned with each patient, taking into account their
exercise capacity tests. 45.
PATIENT MONITORING: patients should be monitored before, during and after the rehabilitaÂtion
session. Variables to monitor are:
0.1 SpO2: it has to be higher than 90% with suppleÂmental
oxygen, with less than 4% variability tolerance during the session.27
0.2 Blood pressure: no more than
20% variability tolerance during the session.46
0.3 HR: no more than 80%
variability tolerance of the TMHR is suggested22 .
0.4 Respiratory rate: it
shouldn’t be higher than 24 bpm.46
0.5 If possible, the session must
be restarted once the already mentioned parameters go back to normal.27
1. MUSCULAR STRENGTH TRAINING:
1.1 It is suggested that patients
begin with big muscle groups (shoulder girdle and pelvic girdle).43
1.2 Then, balance, proprioceptive
and coordinaÂtion exercises will be included. Fall risks will be monitored.27
1.3 Exercise intensity: patients
will begin with acÂtive mobility exercises, and continue with sets of low
intensity exercises using the body-weight (60% of the maximum intensity
achieved with the repetition method), and then will continue to increase
intensity according to the muscular response of each patient. 3 sets per
muscular group with a pause of 2 minutes between each set are suggested.47,48
1.4 Functional training is
recommended.49-51
1.5 A frequency of two times a
day is suggested.27
1.6 Regarding the duration of the
session, it is recommended that the patient begins with 20 minutes and
progresses to 30 minutes per session.
2. AEROBIC CAPACITY TRAINING
2.1 Given the small size of the
rooms in the in-patient ward, exercises should be done with short displacement,
also taking into account epidemiologic safety.
2.2 The intensity of exercise
must be progressive until the patient reaches 80% of the TMHR.
2.3 Training methods can be
continuous or interÂmittent.27
2.4 A frequency of two times a
day is recomÂmended.27
2.5 The duration of the session
shall preferably be 20 minutes, minimum, and must progress to 30 minutes.
RESPIRATORY REHABILITATION AFTER
HOSPITAL DISCHARGE
It is extremely important that
before hospital disÂcharge, a report is made describing the most urgent needs
of the patient, such as the safety of home mobility, symptom control,
supplemental oxygen requirement, suitable nutrition, psychological and social
support, and short- and long-term needs, for example, improvement in physical
and emotional functions and return to work.17
C. RESPIRATORY REHABILITATION IN OUTPATIENTS WITH POST-COVID-19 SYNDROME
AND LONG OR PERSISTENT COVID SYNDROME
This section has the purpose of
addressing reÂspiratory rehabilitation in patients who suffered from the
disease caused by SARS-Cov-2 and were discharged from hospital, as well as
those who were treated on an outpatient basis but evolved and still have
dyspnea.
This chapter uses five trigger
questions about issues of interest to the professionals in charge of the
Respiratory Rehabilitation Programs (RRPs) in outpatient modality.
1. What do post-COVID-19 syndrome and long or persistent COVID syndrome
mean?
In accordance with different
international studies, the duration of the symptoms caused by COVID-19
infection has a mean value of 11 days for patients who weren’t hospitalized and
13 to 25 days for those who required hospitalization52 . However, after the resolution of
the viral infection, it has been observed that some signs and symptoms tend to
prolong. The post-COVID-19 syndrome (hereÂinafter referred to as pCS) is defined as the group of signs and symptoms that
appear after the acute infection has been resolved.53-61 It includes persisÂtent symptoms that could be
related to residual inflammation (in the convalescent phase), organic damage,
non-specific effects of hospitalization or prolonged ventilation (PICS) and
long or persistent COVID (PS).52-53
The first description alerting us
to the imporÂtance of the pCS appeared in a patient
survey conducted in the United States between April and May, 202054. The
name “pCS” came from that work and was endorsed by Greenhalgh in a subsequent publication.55
Spanish authors propose
considering four stages of the SARS-CoV-2 disease and defining those clinical
conditions depending on evolution.56 Thus,
symptoms related to the acute infection would be limited to the first 4 weeks;
acute pCS would describe symptom persistence for 5-12
weeks; proÂlonged symptoms would be divided in two groups: long post-COVID
syndrome (LS), of 12-24 weeks of evolution and persistent syndrome (PS),
prolongÂing beyond 24 weeks from the onset of symptoms.56
However, there isn’t any
universally accepted name in the definitions of pCS
and PS. Two SpanÂish guides define the pCS as the
group of systemic findings beyond 4 weeks from the onset of the first symptom,
with the signs and symptoms being part of the acute infection as essential
requireÂment.52-53 The NICE
Guide (National Institute for Health and Care Excellence) from the United
Kingdom takes the PS into consideration after 12 weeks, and the WHO Guide, as
of the fourth or fifth week.57,58
The frequency of the PS is of
approximately 10-35% of patients in general, even though in critically ill,
hospitalized patients it can reach 80%.53,54,59
2. How to differentiate the pCS and PS from other
similar clinical conditions?
It is important to differentiate
the post-COVID symptoms from other situations that can be simiÂlar but don’t
share their temporal pattern and/or clinical presentation.
A. In cases in which signs and
symptoms are present before the onset of COVID-19 clinical conditions.
B. If signs and symptoms appear after
the infection and weren’t a part of it (post-viral symptoms).
C. If signs and symptoms appear after
the infection and weren’t a part of the initial clinical condiÂtion and
were caused by the organic damage genÂerated by the infection (COVID-19 sequelae).52-53 Unlike the
PS, patients who have progressed with organic sequelae
are usually older males with previous comorbidities that don’t evolve in an outbreak
like the PS.53
D. Finally, the situation arising
from systemic or organic damage due to a severe infection (post- Covid-19
chronic damage)52
3. Which is the presentation and clinical profile of the patient with PS
who is referred to a Respiratory Rehabilitation Program?
LĂłpez LeĂłn et al conducted a systematic review and meta-analysis of the
available literature on prolonged signs and symptoms caused by COÂVID-19
infection.60 6 of
15 studies belonged to hospitalized patients, and they had a follow-up of
14-110 days. 55 persistent signs or symptoms reÂlated to the viral infection
were identified, the most common being: fatigue (58%), headache (44%),
attention disorders (27%), hair loss (25%), and dyspnea (24%). In 7 studies (n
= 1,915 patients), 80% of the subjects had at least one persistent symptom.60
Regarding the profile of the
patient normally referred to RRPs with a diagnosis of PS, a survey of 3,762
patients from 56 countries described sympÂtoms up to 7 months after the onset
of the acute infection.62
Most patients had at least 3
months of evolution, a mean of 14 symptoms per patient and an average of 9
affected organs61.
With respect to the degree of
disability that is usually self-perceived by the patients, a Spanish survey
shows that patients reported 50% disabilÂity.62
When describing each activity in detail,
the most common limitations were found in personal hygiene and daily life
activities, especially family duties and recreation activities.62
4. When, where, and how can a patient with pCS
and PS be initially evaluated?
Evidence regarding which is the
best approach for patients with pCS and PS referred
to the RRP is scarce.30,52,57,58,63-65,66-70 However, there are unaniÂmous criteria about
several important issues.
First, in this work we believe
that patients who have been hospitalized for a long time or had oxygen
requirement or ventilatory support need outpatient or
home respiratory rehabilitation as a continuous strategy following the
treatment that started in the ICU or general ward.
Secondly, given the multiplicity
of organs afÂfected by the PS, the high number of symptoms reported by patients
and their time of evolution, it is necessary to have a multidisciplinary
approach for those who suffered from COVID-19 and arrive at the RRP.30,53,57,58,63-70
In the third place, it’s clear
that, as far as is practical, rehabilitation must focus on the paÂtient.30,53,57,63-70 This means
that the place where the patient is to be evaluated will depend on his/
her needs and possibilities.
A. Remote evaluation of patients
with pCS and PS
Even though there is agreement on
the usefulness of telemedicine in certain groups that apply to the RRPs, at the
moment there isn’t any standardÂized, validated protocol on how to evaluate and
train patients with pCS and PS remotely. The
consulted literature relies on experts’ recomÂmendations.44,52,53,57,63,70 We must take
into account three basic aspects when a patient is going to be included in a
distance RRP: indication, accordÂing to the particular situation of the
patient; the criteria that the patient has to meet in order to access
the intervention on equal terms; the charÂacteristics of the tools that
are going to be used for the evaluation.44,67-70
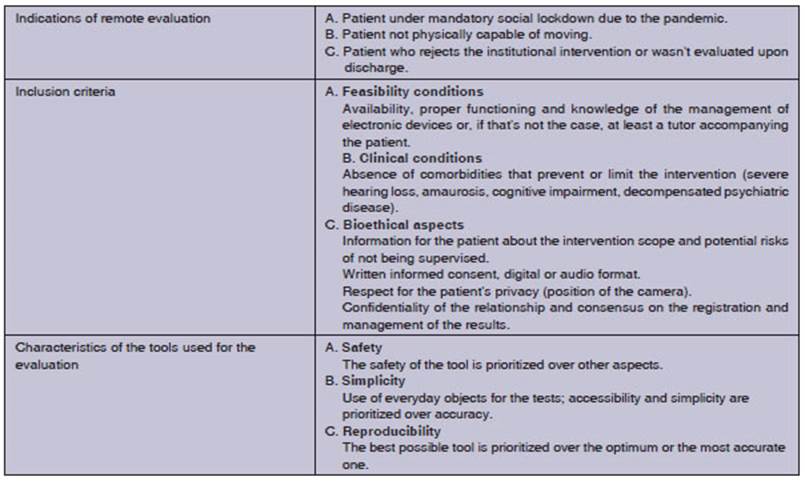
Table 1 describes the
indications, inclusion criÂteria that ensure equality between patients and
tools to be used in the process.
We recommend that the evaluation
of these patients is standardized in steps.
The first step consists in evaluating the patient’s personal history and history of
present illness, provided in the epicrisis of the
hospitalization medical records.30,44,52,53,57,63,66,68-70 The
information to be included is: preexistent comorbidities, history of present
illness, for example, time of evolution of the condition and initial symptoms,
days of hospital stay, extension and severity of the disease, type of oxygen
therapy, if so required (used devices and flows), application, if any, of
invasive and non-invasive ventilation (days of effective ventilation),
administered treatment and patient’s response, laboratory anomalies of clinical
and prognostic relevance and list of complications and potential sequelae registered after hospital discharge.44,66,68,69
The importance of the number of
initial sympÂtoms is related to a higher risk of suffering PS. The presence of
five or more symptoms during the first week of evolution increases the risk of
suffering from a prolonged disease by 3.53 times, compared to patients who show
less than five symptoms.44,64,66,68,69
The second step includes
the remote estimation of the patient’s general condition: his/her aspect, the
state of muscular masses, the ventilatory meÂchanics,
the identification of movement limitations and the state of consciousness69
With the third step we are
able to establish the patient’s level of dyspnea and exercise capacity.
The patient is asked to identify
his/her level of dyspnea in accordance with the Borg dyspnea scale and the
Modified Medical Research Council scale (mMRC).67-70 In order to
test if he/she needs oxygen, the patient is requested to measure oxyÂgen
saturation (SpO2)
while sitting and at rest. If the values are ≥ 96%, the patient is asked
to walk forty steps on a flat surface, with the oximeter.
In the case of patients who don’t have an oximeter,
or as supplementary information of those who do, we recommend exercises that
don’t exceed 4 (four) points in the Borg Scale for perception of dyspnea.69
Apart from estimating dyspnea and
SpO2, the patient’s heart rate (HR) must be monitored, at rest and after each
set of exercises. Since the activÂity isn’t supervised, we suggest the formula
of 220 beats minus the patient’s age.
A second alternative to evaluate
exercise capacÂity is the remote Sit-to-Stand Test (STS). Although it
has been developed and validated for patients with COPD, given its safety and
simplicity, it has been proposed in publications on distance rehaÂbilitation.69-71
From the less demanding modality of 5Rs, to the sit-to-stand in 30 sec (STS30”)
and 1-min sit-to-stand test (STS1’), these tests allow the evaluation of
concentric and eccentric contracÂtion of the quadriceps, the steady state and
even the 1’ variant correlates with the 6-Minute Walk Test (6MWT).65,71
The fourth step consists
in evaluating muscle strength and nutritional status, commonly alÂtered by the sarcopenia of pCS and PICS.30,44,52- 55,57,60,64,66,68-70
We suggest the strength
evaluation method in 8 MRs (maximum repetitions), the evaluation of 3-4 muscle
groups of the upper and lower body and monitoring with the Visual Analog Scale
of HR and SpO2. For the purpose of calculating the patient’s capacity to face
daily activities, we propose evaluÂating the weights using the patient’s body
weight.
With regard to the nutritional
status, the Body Mass Index (BMI) is assessed and muscular masses are observed;
that will allow us to have an approxiÂmate idea on the nutritional status of
the patient.69 Also a virtual follow-up must be performed, and the
nutritionist must provide the most suitable diet for the patient.
The fifth step consists in
evaluating Daily Life Activities (DLAs).
In accordance with the idea of
using the simplest objects for the evaluation, we suggest the use of the
functional status index of patients with COVID, called Post-COVID Functional
Status, at the time of hospital discharge and 4, 8 and 24 weeks after (PCFS).29
The sixth step refers to
the evaluation of the psychological sphere. There is a consensus on the use of
the Hospital Anxiety and Depression Questionnaire (HAD), an instrument that has
been validated for the Spanish language and suggested for virtual PS patients.68,72,73
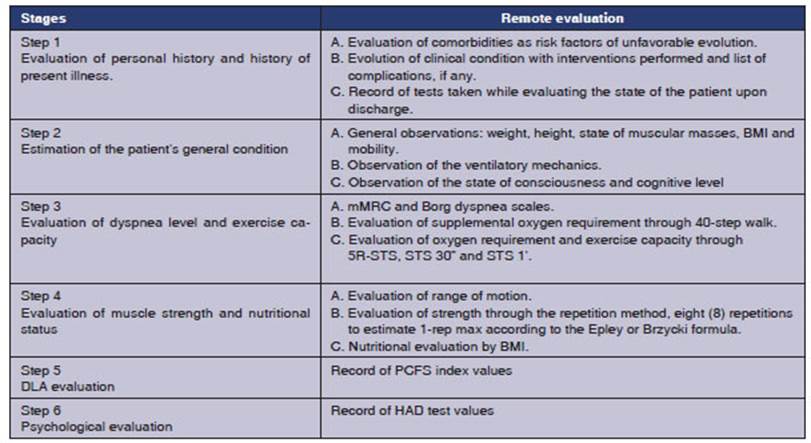
The following table describes the
steps of the remote evaluation of COVID-19 patients.
B. In-person evaluation of
patients with pCS and PS
The in-person evaluation of
patients with pCS and PS shares the first steps with
the remote evaluation, for example, the epicrisis
informaÂtion and general and particular observation of the patient.
With regard to the evaluation of
dyspnea and exercise capacity, with this modality the patient can do the 6MWT
or the Shuttle Test so as to calÂculate those variables and to identify the
impact achieved by rehabilitation.75
To calculate the HR for exercise,
we suggest the Karnoven formula which takes into
account values at rest, heart reserve and maximum reached level.
The tests used to determine which
intensity of aerobic exercise should be indicated are the Incremental Test (IT)
with a treadmill or cycle ergometer and the Constant Load Test (CLT). The IT is
sensitive to interventions and has prognosÂtic implications depending on the
severity of the patient.75 The CLT is the most sensitive tool to
detect the impact of RRPs on respiratory diseases of various origins.75
In the evaluation of muscle
strength and nutriÂtional status, the in-person modality allows the use of
machines, free weight or functional assessment implements such as suspension
straps, exercise balls, bosu balls and body-weight
exercise.44,45
Regarding the nutrition advice,
if the necesÂsary resource is available, it would be desirable to have an
anthropometric measurements form that allows the analysis of the intervention
effects on the patient’s body composition.
For the DLA evaluation we suggest
the PCFS in the first place; the Barthel and Katz
indices can be a second option, and finally, the 36-Item Short Form Health
Survey (SF-36) and the Saint George’s Respiratory Questionnaire (SGRQ) could be
an alternative. The HAD questionnaire can be used for the psychological
evaluation.
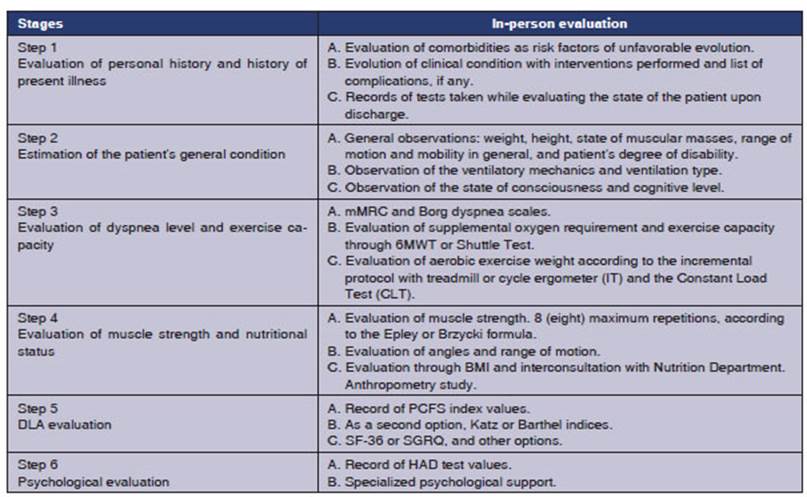
The following table summarizes
the in-person rehabilitation aspects.
3. How to rehabilitate a patient with pCS and
PS?
There isn’t a generalized
consensus on which is the best modality for the rehabilitation of patients with pCS and PS. One concept must
be emphasized in this section.
Several publications
suggest which type of training could be used through telerehabilitation
and in-person rehabilitation, and include not only peripheral muscle training
but also nutritional and psychological support and aspects related to the
patient’s education.30,52,53,57,58,63,66-70
A. Respiratory
rehabilitation with the remote modality
Telemedicine has
provided recommendations for the section about respiratory rehabilitation, both
in the case of an exercise program remotely supervised by a professional and
also in the case of a non-supervised protocol.69,70
Exclusion criteria
for remote respiratory rehaÂbilitation of patients with pCS
are:69
• Poor cognitive
status (Mini-Mental State ExÂamination ≤ 24 points).
• Presence of
unstable heart or neurologic disease.
• Severely altered
range of motion or other musÂculoskeletal defects preventing the patient from
making the requested gestures.
• Disabled patients
who live alone and don’t have any help.
• Patients with
evident balance disorders.
• Patients without
basic knowledge about the management of devices for remote contact.
A1. Asynchronous
remote respiratory rehabilitation
Information about which
type of exercise should be done and how to do it is provided through
videos or workout charts that must be given to the patients. Also, a form must
be given to patients containing all the exercises they have to do. The patient
has to record the level of dyspnea and fatigue he/she felt in each exercise of
the session, according to the Borg scale. If possible, the patient should also
record SpO2 and HR levels at the end of each walk or set of exercises.44,65,69
The educational and psychological
support converge with muscular training to shape this
remote RRP.
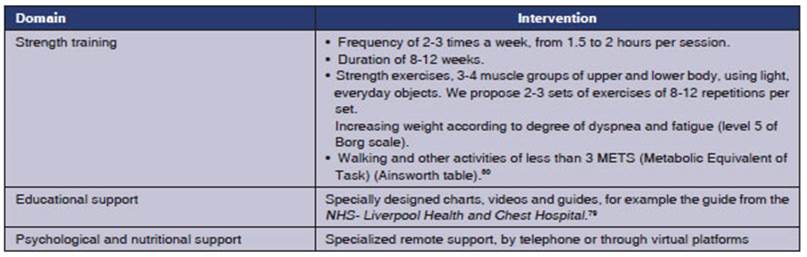
The following table describes the
important aspects of this rehabilitation modality.44,45,66,69,79,80
A2. Synchronous remote
respiratory rehabilitation
With this modality, the
professional can supervise the work of the patient/s in two ways:
On one hand, by connecting to a
video-conferÂence with groups of 4-6 participants and observÂing how they are
doing the activity. On the other hand, connecting individually with the patient
and supervising him/her 2 (two) times a week while he/she does the activity,
leaving other two weekly sessions in charge of the patient himself/ herself.69
B. Respiratory rehabilitation
through the in-person modality
Once the patient finishes his/her
evaluation, the professional has to be able to decide which trainÂing modality
is most suitable for that patient in particular.
B1. Aerobic resistance training
Although there isn’t any specific
protocol for this type of training in patients who suffered from COVID-19
disease, we suggest the training moÂdalities commonly used for patients with
diffuse interstitial lung diseases (DILDs), because they bear some similarity
to the pulmonary damage caused by SARS-CoV-2 and PICS.
In this context, both the
Continuous Variable Method (CVM) and the Intermittent Method can be used.81
A recent update of the Cochrane
Database of Systematic Reviews regarding the RR in DILDs included 16 studies
with 357 DILD patients and a control group of 319 individuals.81 The
rehabilitaÂtion improved the 6MWT with a mean of 40 (± 32.7- 47.4) meters, the
capacity to work, oxygen consumpÂtion, dyspnea and DLAs measured by the SGRQ
and CRQ (Chronic Respiratory Questionnaire), benefits which in five studies
persisted between 6-12 months after finishing the intervention.82
B2. Muscle strength
training
Whether they use
training machines, free weights or functional training elements, patients can
begin muscle strength training with weights that acÂcount for 50% of the
maximal tolerated strength of the evaluation, commonly based on Epley or Brzycki 1-rep max
formulas, then increasing up to 12 reps, and then 3 sets with 80% of maximum
estimated strength.44,81,82
B3. Psychological and
nutritional support
With this in-person
modality, we recommend educational meetings about the aspects related to
posture, dyspnea and cough management in DLAs, breathing rhythm,
energy-conservation techniques when doing physical exercise, suitable use of
canÂnulas and oxygen masks, how to recognize signs of alarm during physical
activities, among other topics of interest.30,44,52,53,55,57,58,63,66,68,69,78,79,81
B4. Psychological
support
This in-person
modality includes a psychopatholoÂgist who is familiar with the problems of
these patients.30,44,52,53,55,57,58,63,66,68,69,78,79,81
CONCLUSIONS
The approach to
patients with moderate and severe forms of SARS-CoV-2 disease involves
recognizing the systemic aspect of the condition, its frequently incapacitating
character and its wide community spread.
At present, the
respiratory rehabilitation is the only intervention that has shown a positive
impact on patients’ dyspnea and fatigue and quality of life, as well as an
improvement in the psychological sphere. Despite those benefits, both the
indicaÂtion and use of respiratory rehabilitation are still strongly
underestimated.
Whatever the medical
complexity level where it is to be applied, we suggest that it is administered
at an early stage, in an integrated and continuous way, during the transfer
from one level of care to another, and in so far as it is possible, with the
participation of a multidisciplinary team consistÂing of kinesiologists,
physicians, nutritionists and psychologists.
Evaluation and
training must focus on the patient’s needs and possibilities. This includes
previous knowledge of the environment where the patient is going to continue
the intervention, that is to say, if it is going to be remote or in-person; the
use of safe and simple techniques with everyÂday objects, the analysis of the
clinical condition of the patient starting the rehabilitation and the
feasibility of the proposed strategy basing on the knowledge of the patient and
his/her environment. Finally, the healthcare team must respect the ethical
principles of privacy, confidentiality and of being informed about the
expectations and results of the suggested intervention.
To conclude, this
workgroup believes that the first duty of the rehabilitation team is to become
the bridge that provides patients affected by SARS-CoV-2 accessibility to the
only valid tool they can have in order to minimize their sequelae
and improve their quality of life: respiratory reÂhabilitation.
Conflict of interest
Authors have no
conflict of interest to declare.
REFERENCES
1.
Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel
Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199-207.
2. WHO Director.General
opening remarks at the media briefing on COVID-19: 11 March 2020. Published March 11 2020. Disponible en: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
3. John Hopkins
Coronavirus Resource Center. Disponible en:
https://coronavirus.jhu.edu/
4.
Las 10 principales causas de defunciĂłn. Disponible en:
https://www.who.int/es/news-room/fact-sheets/detail/the-top-10-causes-of-death
5.
EstadĂsticas-mortalidad. Disponible en: https://www.argenÂtina.gob.ar/salud/instituto-nacional-del-cancer/estadisticas/mortalidad
6.
Ministerio de Salud de la NaciĂłn. BoletĂn Integrado de Vigilancia N540 SE
10/2021. Disponible en: https://bancos. salud.gob.ar/recurso/boletin-integrado-de-vigilancia-n540-se10-2021
7. Schönfeld
D, Arias S, Bossio JC, Fernández
H, GoÂzal D, PĂ©rez-Chada D.
Clinical presentation and outÂcomes of the first patients with COVID-19 in
ArgenÂtina: Results of 207079 cases from a national database. Disponible en:
https://journals.plos.org/plosone/article/authors?id=10.1371/journal.pone.0246793
8. Abate SM, Ahmed Ali S, Mantfardo B, Basu B. Rate of InÂtensive
Care Unit admission and outcomes among patients with coronavirus: A systematic
review and Meta-analysis. PLoSOne 2020;15(7). https://doi.org/10.1371/journal.pone.0235653
9. Osuchowski
MF, Winkler MS, Skirecki T et al. The COVÂID-19
puzzle: deciphering pathophysiology and phenotypes of a new disease entity.
Lancet Respir Med 2021;9:622-42.
https://doi.org/10.1016/S2213-2600(21)00218-6.
10. Hou
YJ, Okuda K, Edwards CE, et al. SARS-CoV-2 reÂverse genetics reveals a variable
infection gradient in the respiratory tract. Cell 2020;182:429-46.e14.
https://doi.org/10.1016/j.cell.2020.05.042
11. Tang X, Du RH, Wang R, et al.
Comparison of hospiÂtalized patients with ARDS caused by COVID-19 and H1N1.
Chest 2020;158:195–205. https://doi.org/10.1016/j.chest.2020.03.032
12. Ellinghaus
D, Degenhardt F, Bujanda L,
et al. Genome wide association study of severe Covid-19 with respiraÂtory
failure. N Engl J Med 2020;383:1522–34.
https://doi.org/10.1056/NEJMoa2020283
13. Yu M, Liu Y, Xu D, Zhang R, Lan L, Xu H. Prediction of the development of pulmonary fibrosis
using serial thin-section CT and clinical features in patients dis charged
after treatment for COVID-19 pneumonia. Korean J Radiol
2020;21:746–55. https://doi.org/10.3348/kjr.2020.0215
14. Wilson MS, Wynn TA. Pulmonary
fibrosis: pathogenesis, etiology and regulation. MucosalImmunol 2009;2:103-21. https://doi.org/10.1038/mi.2008.85
15.
Akoumianaki E, Dousse N, Lyazidi A, et al. Can proporÂtional
ventilation modes facilitate exercise in critically ill patients? A
physiological cross-over study: Pressure support versus proportional
ventilation during lower limb exercise in ventilated critically ill patients.
Ann Intensive Care 2017;7:64.
https://doi.org/10.1186/s13613-017-0289-y
16. Nydahl
P, Sricharoenchai T, Chandra S, et al: Safety of
patient mobilization and rehabilitation in the intensive care unit. Systematicreviewwith meta-analysis. Ann Am Thorac Soc 2017;14:766-77.
https://doi.org/10.1513/AnnalsATS.201611-843SR
17.
Devlin JW, Skrobik Y, GĂ©linas C, et al. Clinical practice guidelines for pain management, agitation/sedation,
delirium, immobility and sleep disturbances in adult patients in the ICU. PADIS Method Innovations Paper. Crit Care Med 2018;46:1457-63.
https://doi.org/10.1097/CCM.0000000000003298
18. Mart MF, Brummel NE, Ely EW. The ABCDEF Bundle for the Respiratory Therapist. Respir Care. 2019;64:1561-73.
https://doi.org/10.4187/respcare.07235
19. Stiller K, Phillips A. Safety
aspects of mobilising acutely ill inpatients. Physiother Theory Pract 2003;19:239-57. https://doi.org/10.1080/09593980390246751
20. Jones PW, Bestall
JC. Modified Medical Research CounÂcil scale. Thorax
1999;54:581-6. https://doi.org/10.1136/ thx.54.7.581
21. Medical Research Council of
the UK, Aids to the investiÂgation of Peripheral Nerve Injuries, Memorandum
No.45. London, Pendragon House 1976: 6-7.
22. Ely E, Truman B, Shintani A, et al. Monitoring SeÂdation Status Over Time in
ICU Patients: Reliability and Validity of the Richmond Agitation-Sedation Scale
(RASS). JAMA
2003;289:2983-91. https://doi.org/10.1001/jama.289.22.2983
23.
Latorre Marco, M. SolĂs Muñoz, T. Falero Ruiz, et. al. ValidaÂtion of the
Scale of Behavior Indicators of Pain (ESCID) in critically ill,
non-communicative patients under mechanical ventilation: results of the ESCID
scale. Enferm Intensiva
2011;22:3-12.
24. Ely EW, Inouye SK, Bernard
GR, et al. Delirium in MeÂchanically Ventilated Patients. Validaty
and Reliability of the Confusion Assessment Method for the Intensive Care
Unit (CAM-ICU). JAMA
2001;286:2703-10. https://doi.org/10.1001/jama.286.21.2703
25. Morris PE, Goad A, Thompson
C, et al. Early intensive care unit mobility therapy in the
treatment of acute respiraÂtory failure. Crit
Care Med 2008;36:2238-43. https://doi.org/10.1097/CCM.0b013e318180b90e
26. Moss M, Nordon-Craft
A, Malone D, et al. A randomized trial of an intensive
physical therapy program for patients with acute respiratory failure. Am
J Respir Crit Care Med 2016;193:1101-10. https://doi.org/10.1164/rccm.201505-1039OC
27.
Ortiz Calderón M, Páez Pineda O. Prevención y Manejo del desacondicionamiento
fĂsico en el paciente hospiÂtalizado por Covid-19. Ed: Universidad PedagĂłgica y
TecnolĂłgica de Colombia. VersiĂłn electrĂłnica. Colombia. Julio 2020.
28. Fuke
R, Hifumi T, Kondo Y, et al. Early rehabilitation to
preÂvent post intensive care syndrome in patients with critical illness: A
systematic review and meta-analysis. BMJ Open 2018;8:1-10.
https://doi.org/10.1136/bmjopen-2017-019998
29. Klok
FA, Boon GJAM, Barco S, et al. The Post-COVID-19 Functional Status scale: atool to measure functional status over time after
COVID-19. Eur Respir J 2020;56:2001494. https://doi.org/10.1183/13993003.01494-2020
30. Barker-Davies R, O’Sullivan
O, Senaratne K, et al. The Stanford
Hall consensus statement for post-COVID-19 rehabilitation. Br J Sports
Med. 2020:54:949-59. https://doi.org/10.1136/bjsports-2020-102596
31. Hong Mei Z, Yu Xiao X, Chen
W. Recommendations for reÂspiratory rehabilitation in adults with coronavirus
disease 2019. Chin Med J (Engl) 2020;133:1595-602. https://doi.org/10.1097/CM9.0000000000000848
32. Wang T, Chau
B, Lui M, et al. Physical Medicine and RehaÂbilitation
and Pulmonary Rehabilitation for Covid-19. Am J Phys
Med Rehabil. 2020;99:769-74. https://doi.org/10.1097/PHM.0000000000001505
33. Stiller K, Phillips A. Safety
aspects of mobilising acutely ill inpatients. Physiother Theory Pract 2003;19:239-57. https://doi.org/10.1080/09593980390246751
34. DĂez
BurĂłn F, Marcos Vidal JM, BaticĂłn
PM. Concordancia
entre la escala verbal numérica y la escala visual analógica en el seguimiento
del dolor agudo postoperatorio. Rev Esp Anestesiol Reanim 2011;5:279-82.
https://doi.org/10.1016/S0034-9356(11)70062-7
35. Núñez-Cortés
R, Rivera-Lillo G, Arias- Campoverde M. Use of
sit-to-stand test to assess the physical capacity and exertional
desaturation in patients post COVID-19. Chron Respir Dis. 2021;18:1479973121999205.
https://doi.org/10.1177/1479973121999205
36. Maddocks
M, Nolan CM, Man WD. Man. Simple functionÂaltests in
COPD: stand up and be counted! Eur Respir J 2017;49:1700104. https://doi.org/10.1183/13993003.00104-2017.
37. Beauchamp MK, Hill K,
Goldstein RS, et al. Impairments in balance discriminate fallers from
non-fallers in COPD. Respir Med. 2009;103:1885-91. https://doi.org/10.1016/j.rmed.2009.06.008
38. Kon
SS, Canavan JL, Nolan CM. The 4 metregait
speed in COPD: responsiveness and minimal clinically important difference. Eur Respir J. 2014;43:1298-305. https://doi.org/10.1183/09031936.00088113
39. Mador
MJ , Mogri M , Patel A. Contractile
fatigue of the quadriceps muscle predicts improvement in exercise perÂformance
after pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2014;34:54-61. https://doi.org/10.1097/HCR.0000000000000023
40.
Ehlenz H, Grosser M, Zimmerann E. La Resistencia desde una Perspectiva Práctica
del Entrenamiento. En: EntreÂnamiento de la Fuerza. 2Âş ed. Ed. MartĂnez Roca
S.A. 1990, p 103-11.
41.
Boeckh-Behrens WU, Buskies
W. Control del esfuerzo según el porcentaje de la fuerza máxima. En:
Entrenamiento de la Fuerza. Editorial Paidotribo.
Barcelona España año 2005, p 64-69
42. Mahoney FI, Barthel DW. Functional
evaluation: the BarthÂel index. Md
Med J. 1965;14:61-65. https://doi.org/10.1037/t02366-000
43. Katz S, Ford A, Moskowitz R, Jackson B, Jaffe
M. StudÂies of illness on the aged. The index of ADL: a stanÂdardized measure
of biological and psychological funcÂtion. JAMA 1963;185:914-9.
https://doi.org/10.1001/jama.1963.03060120024016
44.
RodrĂguez Núñez I, Torres Castro R, Vera R. Consenso de RehabilitaciĂłn
Respiratoria en pacientes con CoÂvid-19. Sociedad Chilena de KinesiologĂa
Respiratoria (SOCHIKIR). Chile. Agosto 2020. https://doi.org/10.13140/
RG.2.2.16594.17607/1
45.
Saadia Otero MA. El Entrenamiento FĂsico en la RehaÂbilitaciĂłn
Respiratoria, un Programa Diferente. Editorial Académica Española. 2017 p
16-19.
46.
Vega ML, Sirotti C, Montiel G, et al. Recomendaciones
para el manejo invasivo y no invasivo de la insuficiencia respiÂratoria hipoxĂ©mica por COVID-19. NĂşmero especial de la Revista Educativa
de ALAT. AsociaciĂłn Latinoamericana de TĂłrax, ALAT. Mayo 2020
47.
Bowers R, Fox E. Procesos de recuperaciĂłn. En:
FisiologĂa del Deporte. 3o ed. MĂ©xico. Editorial MĂ©dica Panamericana, 1998 p
54-69.
48.
Zintl F. Conceptos fundamentales de la teorĂa del
entreÂnamiento. En: Entrenamiento de la Resistencia – FunÂdamentos, mĂ©todos y
direcciĂłn del entrenamiento. 2o ed. Barcelona, España Editorial MartĂnez Roca,
S.A. 1991 p 110-113
49.
Peña G, Heredia Elvar JR, Moral S, Mata F y Marzo Edir Da Silva G. Evidencias sobre los Efectos del
Entrenamiento Inestable para la Salud y el Rendimiento. PubliCE StanÂdard. 2012
50. Willardson
JM. Core Stability Training: applications to sports conditioning programs. J Strength
Cond Res 2007;21:979-85. https://doi.org/10.1519/00124278-200708000-00054
51.
Fajardo J. ¿Qué es la musculación y dónde ubicarla? Nuevas Tendencias en Fuerza
y MusculaciĂłn, 1 ed. Autor Editor Julio Tous
Fajardo, 1999, p 37-5272 273
Respiratory Rehabilitation and SARS-CoV-2
52.
Societat Catalana de Medicina Familiar i ComunitĂ ria (CAMFiC).
Manifestaciones Persistentes de la Covid-19. GuĂa de Práctica ClĂnica. EdiciĂłn
2020.
53.
GuĂa ClĂnica para la atenciĂłn del paciente Long COVID/ COVID Persistente,
1-5-2021. Documento colaborativo enÂtre colectivos de pacientes y sociedades
cientĂficas. VersiĂłn 1.0. Fecha: 1-5-2021.
54 Patient-Led Research
Collaborative. Report: What Does COVID-19 Recovery Actually Look Like? An Analysis of the Prolonged COVID-19 Symptoms Survey by
Patient-Led R Research Team. Disponible en:
https://patientresearchcoÂvid19.com/research/report-1/.
55. Greenhalgh,
T, Knight M, A’Court M, Buxton M, Husain L.
Management of post-acute COVID-19 in primary care. BMJ 2020;370:m3026. https://doi.org/10.1136/bmj.m3026.
56.
Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-
Mayordomo V, Cuadrado ML, Florencio LL. Defining post-COVID symptoms (post-acute COVID, long COVID, persistent
post-COVID): An integrative classification. Int J Environ Res Public Health. 2021;18:2621. https://doi.org/10.3390/ijerph18052621.
57. National Institute for Health
and Care Excellence, PractiÂtioners of RC of G, Scotland HI. COVID-19 rapid
guideline: managing the long-term effects of COVID-NICE Guide (Internet) 2020;
18 December 2020: 1-35. Disponible en:
https//www.nice.org.uk/guidance/ng188/resources/covid19-rapid-guidance-managing-
the-longterm-effects-of-covid19-pdf66142028400325.
58. Rajan
S, Khunti K, Alwan N et al.
In the way for the panÂdemic preparing for long COVID
(Internet) HEALTH SYSÂTEMS AND POLICY ANALYSIS POLICY 2020. Cited 2021
Mar 12. Disponible
en:https://apps.who.int/iris/bitstream/handle/10665/339629/Policy-brief-39-1997-8073-
eng.pdf.
59. Maltezou
H, Pavli A, Tssakris A. Post-COVID Syndrome: An
Insight on Its Pathogenesis. Vaccines 2021;9:497.
https://doi.org/10.3390/vaccines9050497.
60. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50
Long-term effects of COVID-19: a systematic review and meta-analysis. medRxivpreprintdoi: https://doi.org/10.1101/2021.01.27.21250617.
61. Davis HE, Assafi
GS, McCorkelli L, et al. Characterizing Long COVID in
an International Cohort: 7 Months of Symptoms and Their Impact. 2021:101019
https://doi.org/10.1101/2020.12.24.20248802
62.
Sociedad Española de Médicos Generales y de Familia (SEMG). Colectivo de
pacientes Long COVID (ACTS). Encuesta de sĂntomas y discapacidad producida por
los misÂmos, en los afectados por COVID persistente. Disponible en:
https://www.semg.es/images/2020/Noticias/20201111_ReÂsultados_Encuesta_COVID_Persistente.pdf.
63.
Documento intersociedades. DesafĂo pospandemia COVID. Recomendaciones para la rehabilitaciĂłn
pos COVID19. Ministerio de Salud y Bienestar
Social, Argentina.
64. Sudre
C, Murray B, Varsasky t, et al Attributes and predicÂtors
of Long-COVID: analysis of COVID cases and their symptoms 2 collected by the Covid Symptoms Study App.
https://doi.org/10.1101/2020.10.19.20214494.
65. Greenhalgh
T, Javid B, Knight M, et al. What is the efficacy and
safety of rapid exercise tests for exertional
desaturation in covid-19? Oxford COVID-19 Evidence Service.
2020
https://www.cebm.net/covid-19/what-is-the-efficacy-and-safety-of-rapid-exercise-tests-for-exertional-desaturation-in-covid-19/
66. Spruit
MA, Holland AE, Singh SJ, Tonia T, Wilson KC, Troosters
T. COVID-19: interim guidance on rehabilitaÂtion in the hospital and
post-hospital phase from a EuÂropean Respiratory Society- and American Thoracic
Society-coordinated international task force. Eur Respir J 2020;56:2002197. https://doi.org/10.1183/13993003.02197-2020.
67. Brennan D, Tindall L, Theodoros D, et al. A blueprint for telerehabilitation
guidelines. International journal of telerehabilitation
2010;2:31-4. https://doi.org/10.5195/ ijt.2010.6063
68. Vitacca
M, Lazzeri M, Guffanti E et
al. An Italian conÂsensus on COVID-19 patients recovering from acute
respiratory faillure : results of a Delphy process. Monaldi Arch Dis 2020;90:1444:385-93.
https://doi.org/10.4081/monaldi.2020.1444
69. Agency for Clinical
Evaluation. NSW Governement.
DeÂlivering pulmonary rehabilitation via Telehealth
during COVID-19. Virtual
PuRe. April 2020.
Disponible en: www.aci.health.nsw.gov.au.
70.
Almonacid C, Plaza V. GuĂa SEPAR para la teleconsulta de pacientes respiratorios. Disponible en:https://www.separ.es/node/1974.
71. Vaidya
T, Chambellan A, De Bisschop
C. Sit-to-Stand Test on COPD: A literature review. Resp
Med 2017;128:70-7.
https://doi.org/10.1016/j.rmed.2017.05.003
72. Quintana JM, Padierna A, Esteban C, ArosteguiI,
Bilbao A, Ruiz I. Evaluation of the psychometric characteristics of the Spanish
version of the Hospital Anxiety and Depression Scale. Acta
Psychiatr Scand 2003;107:216–21. https://doi.org/10.1034/j.1600-0447.2003.00062.x
73.
Terol Cantero MC, Cabrera Perona V, MartĂn-AragĂłn M.
RevisiĂłn de estudio de la Escala de Ansiedad y DepresiĂłn Hospitalaria (HAD). Anales de PsicologĂa 2015;31:494-503.
http://dx.doi.org/10.6018/analesps.31.2.172701.
74. ATS Committee on Proficiency
Standards for CliniÂcal Pulmonary Function Laboratory. ATS statement:
guidelines for six minute walk test. Am J Respir Crit Care Med. 2002;166:111-17.
https://doi.org/10.1164/ajrccm.166.1.at1102
75.
Puente-Maestu LP, PalangeP,
Casaburi R et al. Use of exerÂcise testing in the evaluation of interventional efficacy : an official ERS statement. Eur Resp
J 2016;47:429-60. http://dx.doi.org/10.1183/13993003.00745-2015.
76.
Alonso J, Prieto L, Anto JM. La versión española del
SF-36 HealthSurvey (Cuestionario de Salud SF-36): un
instruÂmento para la medida de los resultados clĂnicos. Med Clin (Barc). 1995;104:771-6
77. Jones PW, Quirk FH, Baveystock CM. The St George’s reÂspiratory
questionnaire. Respir> Med 1991;85:25-31. https://doi.org/10.1016/S0954-6111(06)80166-6
78.
Curci C, Pisano F, Bonacci
E, Camozzi DM, Ceravolo C, BerÂgonzi R, et al. Early rehabilitation in post-acute COVID-19 patients: data from an
Italian COVID-19 Rehabilitation Unit and proposal of a treatment protocol. Eur J Phys Rehabil
Med 2020;56:633-41. http://dx.doi.org/10.23736/
S1973-9087.20.06339-X.
79. Liverpool Heart and Chest
Hospital. NHS Foundation Trust. COVID-19 Patient
Rehabilitation Guide. https://
www.lhch.nhs.uk/media/7300/covid19-rehabilitation-guide.pdf.
80. Ainsworth B, Haskell W,
Herrmann S et al. 2011 Compendium of Physical Activities: a second update of
codes and MET values. http://dx.doi.org/10.1249/ MSS.0b013e31821ece12.
81. Capparelli
I, Saadia Otero M, Steimberg
J, et al. RehabiliÂtaciĂłn
respiratoria en pacientes con enfermedad pulmonar intersticial difusa,
experiencia de un hospital especializado de Argentina. Rev
Am Med Resp 2019;19:291-7.
82.
Dowman L, Hill CJ, May A, Holland A. RehabilitaciĂłn pulmonar para la enfermedad pulmonarintersticial. Disponible en:https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD006322.pub4/full/es.