Autor : SÃmboli, Norberto FabiÃĄn1,GonzÃĄlez, Claudio Daniel2 TB Diagnostic Study Group Amiano, NicolÃĄs Oscar3; Armitano, Rita InÃĐs4; Bisero, Elsa Delia5; Cerqueiro, MarÃa Cristina6; DurÃĐ, Roberto Miguel7; Fruhwald, Gladys Esther8; GarcÃa, VerÃģnica Edith3; GonzÃĄlez, Claudio Daniel2; GonzÃĄlez, Norma Edith9; Lombardero, Lorena Andrea5; Luque, Graciela Fabiana5; Melillo, Karina Claudia5; SÃmboli, Norberto FabiÃĄn1
1Mycobacteria Service, National Institute of Infectious Diseases - ANLIS Dr. Carlos G. MalbrÃĄn, City of Buenos Aires, Argentina. 2Pneumophthisiology Unit, Hospital General de Agudos JosÃĐ M. Ramos MejÃa, City of Buenos Aires. Argentina. 3Researcher at CONICET (National Scientific and Technical Research Council). Laboratory of Immunity and Tuberculosis of the IQUIBICEN (Institute of Biological Chemistry, Faculty of Exact and Natural Sciences), University of Buenos Aires (UBA), City of Buenos Aires. Argentina. 4Laboratory for Mycobacteria. Hospital General de Agudos Parmenio P. PiÃąero. City of Buenos Aires. Argentina. 5Pediatric Service. Pediatric Pulmonology Department, Hospital Nacional Prof. Dr. Alejandro Posadas. El Palomar, Province of Buenos Aires. Argentina. 6Consulting Physician in the Department of Phisiology. Hospital de NiÃąos Dr. Ricardo GutiÃĐrrez. City of Buenos Aires. Argentina. 7Bronchoscopy Unit, Hospital de Infecciosas Francisco J. MuÃąiz. City of Buenos Aires. Argentina. 8ulmonology Service of OSPERYH (Health Insurance for Rental and Horizontal Property Workers). 9Pneumophthisiology Unit, Hospital General de NiÃąos Pedro de Elizalde. City of Buenos Aires. Argentina.
https://doi.org/10.56538/ramr.WNVT5636
Correspondencia : Claudio Daniel GonzÃĄlez (claudiodgonzalez57@gmail.com)
Recibido:
11/17/2021
Aceptado:
03/17/2022
DIAGNOSIS OF
TUBERCULOSIS
Claudio
D. GonzÃĄlez
One of the main challenge for Tuberculosis Control Programs (TCPs) is early
detection of open forms of the disease. The World Health Organization (WHO) has
estimated that the development of a tuberculosis (TB) diagnostic method that
offers 85% sensitivity and 95% specificity in sputum samples would allow saving
around 400,000 lives per year.1 Under ideal
conditions, it would also be necessary to have an affordable and precise method
applicable to the most vulnerable groups which contributes to the
identification of the species and its resistance profile, especially in cases
that imply a higher risk of therapeutic failure.1
In the last decade, the development of the GeneXpert
MTB/RIF diagnostic system has been a major breakthrough in that regard. At a
cost of USD 9.98 per determination (in the 145 subsidized countries), the
method helped get closer to the mentioned objectives, that is to say, early
detection of TB and detection of resistance to rifampicin, usually considered
an indicator of therapeutic failure.2-4
Unfortunately, the emergency of
the SARS-CoV-2 pandemic impacted negatively on the achievements. Two aspects of
TB patientsÂī care were affected: one, the regular and complete provision of
supplies for the diagÂnosis and treatment of the disease; the other aspect was
related to delays and postponement of consultations caused by lockdown measures
and social
The purpose of this work was to
review the current state of knowledge of valid TB diagnostic methods. To make
reading easier, this updating document has been divided into three publicaÂtions.
This first publication includes the diagnosÂtic methods aimed at identifying
the causative agent and its sensitivity profile, that is to say, the bacteriologic
or certainty diagnosis. The second publication will address the methods
that evaluate the host response to the bacillus, in other words, the non-bacteriologic
or presumptive diagnosis, which includes some methods that are still under
investigation. The third publication will refer to the diagnosis of TB in
children.
BACTERIOLOGIC DIAGNOSIS OF TUBERCULOSIS
Norberto SÃmboli
The National TB Diagnostic
Laboratory Network is a pyramid organizational structure where each level has
specific infrastructure and biosafety reÂquirements defined by the activities
and diagnostic methods performed in each laboratory. As the laboÂratory level
increases (1 to 3), the technologies get more innovative; as a result, the
personnel need to have more abilities and higher competence, and training
requirements increase.6
Diagnostic methods are classified
based on the three laboratory levels, according to the risk level associated
with each procedure, the epidemiologiÂcal situation of the disease and the
available resouÂrces.6 Following
such classification, this document has the purpose of reviewing the current
state of knowledge about available techniques and offering an initial approach
to methods of bacteriologic diagnosis which are still under development. The
complexity levels mentioned before are:
1. FIRST LEVEL OF COMPLEXITY
It includes peripheral
laboratories located, in some cases, in health centers offering direct test by
sputum (DT) through the Ziehl Neelsen
(ZN) teÂchnique and Kudoh-Ogawa (KO) culture medium.
At present, the GeneXpert MTB-RIF, TB-LAMP (loop-mediated isothermal
amplification) and LF-LAM (lateral flow lipoarabinomannan
assay) diagnostic methods are being included.6
2. SECOND LEVEL OF COMPLEXITY
This level includes laboratories
of local or reÂgional hospitals with the capacity to do all level 1 activities
plus solid- or liquid-medium cultures, identification of the Mycobacterium
tuberculosis (M. tuberculosis) complex and sensitivity tests (STs) for
first-line drugs (isoniazid and rifampicin), plus those with the capacity to
perform FL-LPA (line probe assay for first-line drugs) and SL-LPA (line
probe assay for second-line drugs), always from sputum samples with
positive bacilloscopy.6
3. THIRD LEVEL OF COMPLEXITY
It consists of national or
provincial reference laboÂratories, or specialized laboratories that have the
resources to carry out all the studies of the two previous levels plus STs for
second-line drugs and complex molecular techniques.
1. FIRST LEVEL OF COMPLEXITY
Direct test (DT)
For some time, countries with
limited resources have used the microscopy as the main method to detect M.
tuberculosis. Although the DT is low-cost and requires minimum biosafety conditions,
it has limited sensitivity, especially in patients living with HIV/AIDS and
children under 5 years, and it doesnât provide information about the drug
resistance profile of bacilli.7 Despite the
fact that the microscopy is not able to differentiate M. tuberÂculosis from
other mycobacteria, in countries with TB endemic, the positive bacilloscopy of a respiÂratory sample from an immunocompetent patient has very high predictive value for
TB diagnosis.7
ZN staining has been the most
widely used teÂchnique for TB diagnosis in Latin American counÂtries.7 Compared to
fluorescence microscopy (FM), the conventional
microscopy has the advantage that it requires less training, because it is
easier to acquire the capacity to identify the bacillus through this
methodology. Also, the DT through ZN staining is still a useful resource in our
counÂtry for TB screening in patients with respiratory Revisymptoms
(RSs), that is to say, people with cough and expectoration for
more than two weeks.7
In 2011, the WHO (World Health
Organization) recommended the use of the FM with LED light. The FM is at least
20% more sensitive than conÂventional microscopy through ZN.8
Given that it reduces the time necessary for the reading and
requires trained personnel, it is especially recomÂmended for laboratories with
heavy workloads. In comparison with conventional FM (with a mercury lamp), the
FM with LED light offers considerable operational advantages because it has a
long-life span, it doesnât generate heat and doesnât involve environmental
pollution risks if it breaks. If a center uses this method instead of ZN
staining, it must meet the technical requirements demanded by the WHO and the
corresponding external quaÂlity monitoring.8
For the past few years, rapid and
sensitive tests have been available; such tests are based on molecular methods
to replace or compleÂment the microscopy.
Kudoh-Ogawa culture method
Laboratories without the
necessary conditions for culturing through methods that require centrifuÂgation,
which are located far away from a reference laboratory or donât have a regular
sample transÂportation system can inoculate the samples with the Kudoh-Ogawa method and send the inoculated tubes to the
reference laboratory.9
GeneXpert MTB/RIF- MTB/ULTRA- Xpert XDR methods
The development of the XpertÂŪ MTB/RIF assay
for the GeneXpert platform was completed in 2009, and
is considered an important breakthrough in the fight against TB. For the first
time, a molecular test was simple and robust enough to be introduced and used
outside the conventional laboratoriesâ environment.10
It detects the Mycobacterium
tuberculosis comÂplex (MTBC) and also the most common mutations that confer
resistance to rifampicin using three specific primers and five unique molecular
probes to ensure a high degree of specificity. It is a closed automated system
of real-time extraction and amÂplification. It allows the detection of the MTBC
in a great amount of clinical samples in 2 hours, with a detection limit of 114
ufc/mL, and reasonable conditions of accessibility,
cost and security.2,
3, 10
It is a rapid, simple test that
can be used in laboratories with minimum infrastructure, and allows for an
increased number of detected TB caÂses, compared to the microscopy. This is
favorable in terms of reducing investment in infrastructure and equipment for
health services. There are also other benefits for public health, such as the
poÂtential reduction in the secondary transmission of resistant strains, an
aspect that assumes especial importance within the context of a
multidrug-resistant tuberculosis epidemiology (MDRTB) in our country, where the
greater MDRTB-generating impulse is associated with strain transmission in the
community.11
When different studies evaluated
the overall pooled sensitivity-specificity of lung samples compared to the DT,
it was shown that their perÂformance reached 88% and 99%, respectively.2-4 In positive sputum DT/culture samples, sensitivity was 98%,
whereas in negative DT/positive culture samples, pooled sensitivity was 80%.
This perÂformance, replacing the DT as the initial test or in comparison with
negative DT samples, would allow 30% detection improvement through baciÂlloscopy, compared to the ZN technique, and would
considerably reduce the time since the beginning of treatment.12
This performance also includes the group of patients living with
HIV, in which it doubles the TB detection rate and reaches a global performance
of 79%.2, 3
With the same cartridge, the
system offers a seÂcond use, which is to do the sensitivity test (ST) for
rifampicin, reaching a pooled sensitivity-specificity of 95% and 99%,
respectively.2,
3
On the other hand, in extrapulmonary samÂples of adults and children, the highest
pooled sensitivity-specificity obtained with Gene-Xpert,
compared to the culture samples, was found in ganglion samples (84.9% and
94.2%, respectively), followed by gastric lavage and aspirate samples (83.8%
and 98.1%, respectively), cerebrospinal fluid (79.5% and 98.6%), and, at last,
pleural liquid (43.7% and 98.1% pooled sensitivity and specificity,
respectively).2,
3
The XpertÂŪ MTB/RIF Ultra
has been developed as a new generation assay to overcome the limiÂtations of
the Xpert MTB/RIF, and uses the same GeneXpertÂŪplatform.13
In order to improve sensitivity
for detecting the MTBC, the Xpert Ultra incorporates
two diffeÂrent multicopy amplification targets
(IS6110 and IS1081) and one DNA reaction chamber bigger than Xpert MTB/RIF (PCR [polymerase chain reaction] of 50 μL in the Ultra versus 25 μL in Xpert MTB/RIF).13
It also incorporates nested-like
nucleic acid amplification, faster thermal cycling and improved fluids and
enzymes. This resulted in the Xpert UlÂtra having a
detection limit of 16 ufc/mL (compared to 114 ufc/mL for the Xpert MTB/RIF). In order to improve the accuracy of
rifampicin resistance detection, the Ultra incorporates an analysis based on
the melting temperature instead of a real-time PCR. Specifically, four probes
identify rifampicin resistance mutations in the determining region of the rpoB gene and move the melting temperature away from the
reference value of the wild type.13
Investigation of TB with MTB/RIF
cartridges has consistently proven to be more sensitive than bacilloscopy. The Ultra version of the Xpert
MTB/ RIF cartridges is even more sensitive, especially in negative DT samples,
positive cultures, and samples of HIV patients, but less specific than the
previous version, mostly among patients with history of TB treatment.9
Patients with TB and rifampicin
resistant TB (RRTB) should promptly do additional tests for detection of
resistance at least to isoniazid and fluoroquinolones,
respectively, so as to guide treatment decisions.
In 2020, the WHO requested a
systematic reÂview of published and unpublished data regarding three classes of
nucleic acid amplification tests that hadnât been previously reviewed by that
orÂganization.14 One of them
was the new MTB/XDR cartridge, which showed excellent sensitivity and
specificity to rapidly detect resistance to isoniazid, fluoroquinolones
and aminoglycosides.
Recently, the WHO recommended the
use of the cartridge for the rapid detection of mutations that confer
resistance to these drugs.14 This recommenÂdation was based on the analysis of three
studies including sputum samples of 1605 participants. This analysis showed
that the overall combined sensitivity of this cartridge (95% CI [confidence
interval]) for the detection of resistance to isoniazid was 94.2% (89.3% to
97.0%), and the specificity was 98.0% (95.2% to 99.2%). The overall combined
sensiÂtivity (95% CI) for the detection of resistance to fluoroquinolones
was 93.1% (88.0% to 96.1%), and the specificity was 98.3% (94.5% to 99.5%). The
global combined sensitivity (95% CI) for the detection of resistance to amikacin was 89.1% (80.9% to 94.1%), and the specificity
was 99.5% (96.9% to 99.9%). The phenotypic ST was used as standard of reference
for the three estimations mentioned before. The overall sensitivity (95% CI)
for the detection of resistance to ethionamide was
96.4% (92.2% to 98.3%), and the specificity was 100.0% (82.5% to 100.0%). The
gene sequencing of the inhA promoter
region was used as standard of reference for the detection of resistance to
ethionamide.14
The Xpert
system, in all its forms of presentaÂtion, can be used in low-complexity
laboratories, under the same conditions required to perform a bacilloscopy.
Recommendations for the use of Xpert MTB/ Rif and ULTRA:
The WHO recommends the use of the
Xpert MTB/RIF and Xpert
Ultra as initial tests in adults and children with signs and symptoms of pulmoÂnary
and extrapulmonary TB basing on current scientific
evidence.15
Given the fact that when this
consensus was achieved our country had limited access to rapid molecular tests,
due to the restricted availability of the Gene-Xpert
equipment, this test is recomÂmended mainly for the following groups:
I. Adult or pediatric patients
with high clinical and epidemiological suspicion of TB and risk of multiresistant TB (strong recommendation).
II. Adult or pediatric patients
with high clinical and epidemiological suspicion of TB or MRTB living with HIV
(strong recommendation).
III. Adult or pediatric patients
with high clinical and epidemiological suspicion of meningeal TB (strong
recommendation).
IV. Adult or pediatric patients
with high clinical suspicion of extrapulmonary TB
(conditional recommendation).15
Its use is indicated in the
following cases:
a) Respiratory samples of adult
or pediatric paÂtients who show signs and symptoms compatible with TB and
higher risk of suffering resistant TB: people living with HIV, immunosuppresÂsed
patients, healthcare personnel, contacts of patients with RRTB or MRTB, and
patients who completed treatment with antituberculous
drugs more than 1 year before.
b) Cerebrospinal fluid samples,
lymph node aspiraÂtion, synovial fluid, pleural liquid, peritoneal fluid,
pericardial fluid, urine and biopsies from adult or pediatric patients with
high clinical or epidemioloÂgical suspicion of extrapulmonary
TB.15
These recommendations are
periodically reÂviewed, basing on technological development and the existence
of scientific evidence that justifies such review.
The use of the Xpert MTB/XDR cartridge is recommended in sputum samples of
patents with RRTB.
Truenat MTB, MTB Plus and MTB-Rif DX
These are new molecular methods,
developed in India, which may be used on the same laboratory level as the Gene-Xpert. They are based on a real-time micro-PCR that allows
for the detection of the MTC and its resistance to rifampicin from a sputum
sample in less than 1 hour. The Truenat MTB and the
MTB Plus can be used as initial diagnostic tests in adults and children with
signs and symptoms of pulmonary TB, whereas the MTB-RIF Dx
is used to detect resistance to riÂfampicin in samples that had positive
results in the initial test.16
TB-LAMP (loop-mediated isothermal
amplification)
TB-LAMP is a commercial molecular
assay based on loop-mediated isothermal amplification which requires minimum laboratory
infrastructure and biosafety requirements.17
It has been evaluated as a rapid test (<2 h) at the point of
care testing as an alternative to the sputum DT, which is still the primary
diagnostic test for pulmonary TB in limited resource environments.
In January 2016, the WHO
organized a meeting with the Guideline Development Group (GDG), to review the
evidence published from 2012 to that moment.17
The review included all prospective studies evaluating the
TB-LAMP assay in spuÂtum samples of adults with signs and symptoms compatible
with pulmonary TB that were carried out in environments with high or
intermediate TB burden. In this review, which included twenty stuÂdies (4760
adult patients), the TB-LAMP showed a combined sensitivity 15% higher than the
DT to detect pulmonary TB in adults (78% versus 63%),
though the combined specificity was 2% lower (98% versus 100%). This can partly
be explained by the identification of TB cases wrongly classiÂfied as negative
TB through the use of reference cultures. The evaluation of TB-LAMP accuracy in
adults living with HIV with signs and symptoms of pulmonary TB showed
sensitivity and specificity percentages similar to those of sputum DTs (64% and
62%) and (99% and 99%), respectively.17
In accordance with this evidence
evaluation, and taking into account the costs and benefits associated with the
use of TB-LAMP, the WHO recommends it for use only in sputum samples in one of
these ways:
a) As an alternative to the bacilloscopy for the diagnosis of pulmonary TB in adults
with signs and symptoms compatible with TB (conditional recommendation, very
low- quality evidence).
b) As additional test, apart from
the DT, in adults with signs and symptoms compatible with pulmonary TB,
especially in cases of negative sputum DTs (conditional recommendation, very
low-quality evidence).
In our country, this method isnât
available yet.
LF-LAM (lateral flow lipoarabinomannan
assay)
Tests based on the detection of
the mycobacterial lipoarabinomannan (LAM) antigen in
urine have emerged as potential rapid tests at the point of care testing for
the diagnosis of TB.18 The LAM
antigen is a lipopolysaccharide present in the cell walls of mycobacteria,
which is released from metabolically active or degenerating bacterial cells and
seems to be present only in patients with active TB. This test would be better
than sputum-based tests beÂcause urine is easy to collect and store and doesnât
entail the infection control risks associated with sputum collection.18
LAM detection assay in urine
through lateral flow immunochromatography is
commercially available. The test is done manually, applying 60 μL of urine to the reagent strip and incubating at room temperature for 25
min. Then the strip is inspected visually. The intensity of any of the bands
visible in the reagent strip is classified, comÂparing it with the band
intensities of a reference card provided by the manufacturer.18
Several studies and meta-analyses
of a previous generation test (LAM-ELISA) have shown good sensitivity for
detecting urinary LAM in cases of HIV-TB coinfection,
and sensitivity increaÂses even more with lower LTCD4+ counts. This finding contrasts with
traditional diagnostic methos for TB in patients with
HIV. Several hypotheses can explain the higher sensitivity of LAM detection in
urine in patients with HIV-associated immunosuppression: higher bacillary and
antigen burden, higher probability of having TB in the urogenital tract and
higher glomerular permeability to allow increased levels of antigen in the
urine.18
Some published studies reported
much higher mortality rates in patients with HIV with low LTCD4+ counts who have detectable urinary LAM,
compared to individuals with negative LF-LAM results.18
Given the potential of the assay to help reduce mortality in
patients living with HIV and the fact that the test is easy to do and requires
minimum biosafety infrastructure, the WHO requested a systematic review of the
use of the LF-LAM assay for the diagnosis and detecÂtion of active TB in people
living with HIV. After that review, the organization made the following recommendations
on the use of this assay:15, 18
a) Except for people with HIV
infection who are seriously ill or have low LTCD4+ counts, the LF-LAM SHALL NOT be used for the
diagnosis of TB (strong recommendation, low quality evidence).
b) LF-LAM can be used to help
diagnose TB in HIV patients with signs and symptoms of TB (pulmonary or extrapulmonary) with a LTCD4+ cell count lower than, equal to or higher
than 100 cells/μL, or HIV-positive patients who are seriously ill regardless of their
LTCD4+ count,
or with an unknown count (conditional recomÂmendation, low quality evidence).
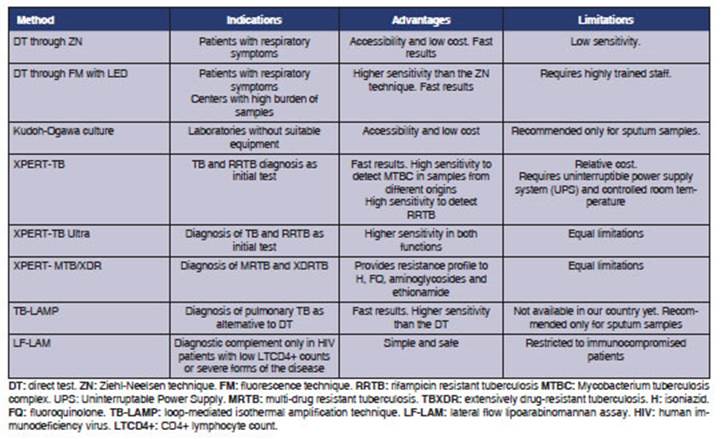
Table 1 summarizes the diagnostic
methods related to this level.
2. SECOND LEVEL OF COMPLEXITY
Culture
As we mentioned before, the DT is
still the priÂmary diagnostic test for pulmonary TB in limited resource
environments.
The culture complements the DT in
that it allows us to show viable bacilli present in low amounts in a lesion
sample, to characterize them and know whether they are sensitive or resistant
to antituÂberculous drugs. The role of the culture is
more important in a context of medium or low incidence of TB, with a high
incidence of TB bacillus/HIV coinfection, and medium
or high MRTB burden.19
Through the culture, it is
possible to increase the number of cases with confirmed diagnosis of TB in
approximately 15%-20% of the total number of cases, and 20%-30% of the cases
with pulmonary TB. If we take into account the total number of caÂses with a
bacteriologically confirmed diagnosis of pulmonary TB, the bacilloscopy
detects 70%-80% of the cases, and the culture detects the remaining 20%-30%.19
The solid-medium culture is still
being used as a reference point for the more modern, automated liquid media,
and continues to be the reference method compared to other diagnostic systems.
The solid medium has the advantage of being low-cost, but takes more time to
detect bacterial growth.
Liquid-medium culture
methods
The main advantage of these
systems, compared to the traditional culture, has to do with their fast reÂsults.
They use a colorimetric system to inform about bacterial growth (MB Bact Alert) or the detection of consumed oxygen by
fluorescence (MGIT 960). For blood and bone marrow samples, the lysis-centrifuÂgation technique for blood cultures is
applied.7
The biosafety conditions required
by methods are different from those of solid media; they can be used on this
level of complexity only if such conditions are observed.
Culture indications
a) Given the fact that lesions in
children are usually paucibacillary, there is a
strong recomÂmendation that all pediatric samples should be cultured, because
they increase the DT performance by 20%.3 The following respiratory
samples are indicated, in order of preference: sputum, gastric aspirate in RS
children with pathological chest X-ray (Rx), induced sputum, bronchial aspirate
and bronchoalveolar lavage; among non-respiratory
samples, the content of serous cavities and biopsies.3
b) Samples of symptomatic
patients with clinical signs, Rx or other images compatible with TB and one of
the following characteristics:
âĒ Negative bacilloscopy
of three respiratory samÂples.
âĒ Extrapulmonary
localization of the disease.
âĒ Immunosuppressed patients,
particularly HIV positive individuals.
âĒ Positive bacilloscopy
in gastric lavage, bronchial lavage or swabs.
âĒ History of anti-tuberculous treatment, espeÂcially in cases of loss to
follow-up or treatment failure.
âĒ Exposure to infection by
drug-resistant bacilli (contact with cases of resistant TB, hospitalized
patients or workers of health institutions or prisons with registered cases of
MRTB).7, 19
âĒ To complement rapid diagnostic
tests when they are used as the initial diagnostic test.
First-line drugs sensitivity
testing (H and R) Phenotypic tests.
On this level of complexity, the LÃķwenstein-Jensen medium proportion method (method of
Canetti, Rist and Grosset)
still provides the well-known simplicity and reliability for which it has been
considered the method of reference, compared to molecular-based genotypic
methods. It is an economical method, but it has the disadvantage of taking 30
to 40 days to obtain a sensitivity result.
An economical alternative to
accelerate results is the nitrate reductase assay
(NRA). Ideally, the method shall be used directly with positive DT samples
collected at the moment or as soon as the primoculture
is developed. This test is supported by the WHO, for being considered an
accessible and effective ST for determining resistance to isoniazid and
rifampicin.19
A more expensive alternative is
the use of liquid-medium cultures (MGIT) that accelerate results because they
use semi-automated equipment that detects bacterial growth before it is
visible.19
ST indications
Ideally, all cases with
bacteriologically confirmed diagnosis of TB must have access to the ST, at
least for drugs that are crucial to treatment success (H and R). Universal
access to recommended rapid tests shall be guaranteed (Xpert5, LPAS,
etc.). In the process of achieving this objective, the ST should be the
priority in cases with the following characteristics, which increase the risk
of drug resistance.7
a) Treatment failure.
b) History of previous treatment,
irregularity in treatment compliance or prescription of an incomplete or
inadequate regimen.
c) Exposure to infection by
drug-resistant TB.
d) Children.
e) Immunosuppressed patients
(people living with HIV and/or diabetes, etc.).
f) Previous residence in
countries with a high burden of drug-resistance (Ecuador, Peru, some Asian and
East European countries).
g) Drug or alcohol abuse.
New platforms
New technologies for rapid
detection of TB and resistance to rifampicin are becoming more and more
available and are being adopted by several countries. Several manufacturers
have developed automated platforms for detecting TB
and resisÂtance to rifampicin and isoniazid (Abbott, Becton Dickinson, Roche, Hain Lifescience/Bruker) basing on nucleic acid amplification.20
These tests are
faster and less complex than the phenotypic drug-sensitivity tests based on
cultures and line probe assays (LPA). They have the advanÂtage of being mostly
automated, and may be used as the initial test to detect TB and resistance to
both first-line drugs simultaneously (rifampicin and isoniazid). They offer
fast and accurate results and process a large number of samples; thus, they are
adequate for laboratories of medium and high burden of sensitivity tests. So,
these technologies are suitable for high-density population areas and fast
sample reference systems.20
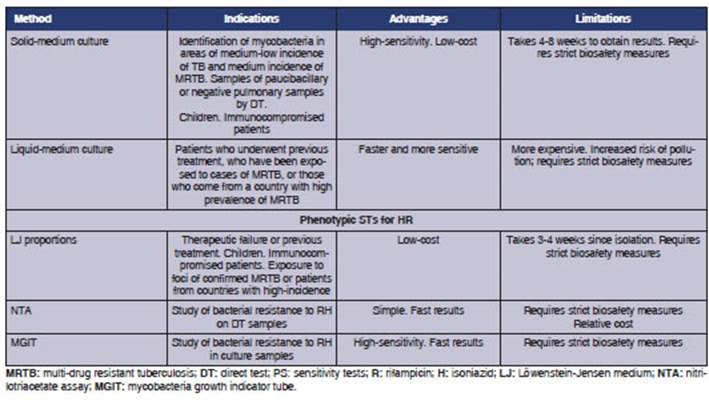
Table 2 summarizes
the diagnostic methods related to this level.
3. THIRD LEVEL OF
COMPLEXITY
Species
identification. First-
and second-line drugs sensitivity testing
Phenotypic tests for
species identification
Apart from the
traditional system used for species identification in solid-medium cultures,
there is a lateral flow immunochromatography that
idenÂtifies the M. tuberculosis complex qualitatively from positive
liquid-medium cultures. This system detects a protein (MPT64) that is
secreted by the bacteria into the culture medium. It is a simple, fast,
low-cost, high sensitivity-specificity techniÂque. The disadvantage of this
technique is that it canât differentiate the species of that complex, and the
results must be contextualized with the clinical information.19 It can also be used for the identification of MTBC in
positive solid-medium cultures.
First- and
second-line drugs phenotypic sensiÂtivity testing
Indirect ST through
the proportion method using solid media is the most common method to show the
sensitivity of M. tuberculosis isolates.
The BACTEC-MGIT
system is the preferred method for the ST of many antibacillary
agents, given the standardization of MGIT media and instruments. This system of
automated reading is the most widely used on this level of complexity, because
it considerably reduces the time of detecÂtion of rifampicin and isoniazid
resistance, with 95%-98% sensitivity.2 The disadvantage regarding
detection of resistance to ethambutol, pirazinamiÂde and streptomycin (this drug is no longer used
for the treatment, but sometimes itâs necessary to include it) lies in its low
reproducibility; so, it is used for national reference laboratories that have
other methods to confirm results related to these drugs. Indications were
already described before.
Genotypic
sensitivity testing (line probe assay, LPA)
The amplification and
detection of the nucleic acids of the M. tuberculosis complex is a
technology that has proven to be very sensitive and specific. Some
amplification techniques have the advantage of being able to detect resistance
to certain antituÂberculous drugs.21
Real-time PCR applied
to some tools is the most widely used technique at present. These tools deÂtect
the DNA of the Mycobacterium tuberculosis complex and distinguish gene
mutations related to drug resistance. Generally speaking, they have a
considerable cost and require staff training in order to meet the requirements
of international standards and external audits.19, 21
The LPAs are a group
of tests based on multiplex PCR and strip-based reverse hybridization. They
amplify gene segments where the most frequent mutations that originate
resistance are produced.21
They also amplify a
specific segment of the M. tuberculosis complex, so it is also possible
to detect the complex. The resistance of M. tuberculosis rifampicin and
isoniazid-resistant isolates, which is 5% and 15% ,
respectively, may not be detected by these systems because they have genetic
alteÂrations in regions that are not covered by them.
The LPAs are
technically more complex than the Xpert MTB/RIF-ULTRA
or XDR assays; however, they can also detect resistance to a variety of
first-and second-line agents (for example, isoniazid, fluoroquinolones
and injectable drugs), and their results can be obtained in 24 hours.
There are two large
groups of assays:21
âĒ Those which detect
MTC and resistance to first-line antituberculous
agents (known as first-line LPA [FL-LPA]), as for example, GenoType
MTBDRplus v1 and v2, Genoscholar
NTM + MDRTB II.
âĒ Those which detect
resistance to second-line antituberculous agents
(known as second-line LPA [SL-LPA]), as for example, GenoType
MTBDRsl.
The use of the LPA to
detect resistance doesnât eliminate the need to do a conventional culture and
the phenotypic ST, since they have a critical role in the follow-up of
treatment response and the detection of additional resistance to other drugs.7
In general, the LPAs
canât be used as the initial diagnostic test as a replacement for the DT
because they have limited sensitivity and have to be done in laboratories with
a specific level of complexity.21
Given the increasing
incidence of MRTB, the LPA system has been evaluated to detect or discard MRTB
and extensively-drug resistant tuberculosis (XDRTB).
Other molecular
techniques for the identificaÂtion of species or their clonality
The next-generation
sequencing (NGS) has great potential as a method for the rapid diagnosis of
drug-resistant tuberculosis (DRTB) in various environments of clinical
reference laboratories throughout the world.22
The NGS approach
overcomes many of the important challenges associated with conventional
phenotypic tests, as well as the limitations of other less complete molecular
tests, by providing fast and detailed information of sequences for multiple
gene regions or complete relevant genomes. However, the use of these
technologies for the diagnosis of DRTB has been obstructed due to elevated costs,
integration in the laboratory workflow, technical training requirements
necessary to use the techÂnology, and the need of expert guidance for clinical
data management and interpretation.22
Other complex
diagnostic methods aim at knowing about the transmission of the disease within
the community; this can be achieved by identifying the clonality
of species through molecular techniques, such as RFLP (restriction fragÂment
length polymorphism) or by whole genome sequencing techniques (WGS). In all
cases, its use is restricted to central reference laboratories.22
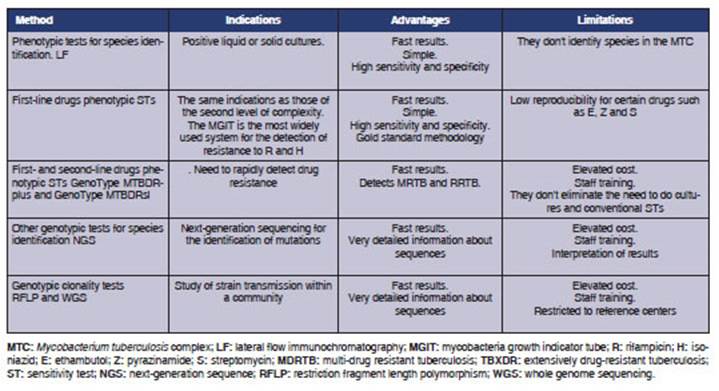
Table 3 summarizes
the diagnostic methods related to this level of complexity.
CONCLUSIONS
The strategy and
goals for the prevention, care, and control of tuberculosis after 2015, briefly
called âAn end to TBâ, approved by the 67th World Health Assembly in May 2014
through resolution WHA 67.1, and launched by the WHO, proposes a TB control
approach that goes beyond the healthÂcare sector. It takes into account
biological factors and socio-economic conditions that define which
are the populations with higher risk of suffering TB, as well as the strengths of the research on new vaccines, diagnostic
methods, and drugs that will make way for the elimination of this disease.
Recent discoveries
about the diagnosis of TB provide an opportunity to improve the capacity of the
laboratories to reach an early and accurate diagnosis of sensitive and
resistant TB. One of the most important elements for adopting these new
technologies is the existence of diagnostic policies that incorporate these
techniques in the diagnostic algorithms and establish training plans and
external evaluations of the quality of said techniques.
Conflict of interest
The authors of this
work have no conflicts of interest to declare.
REFERENCES
1. McNerney
R, Maeurer M, Abubakar I,
et al. Tuberculosis Diagnostics and Biomarkers: Needs, Challenges, Recent
Advances, and Opportunities. J Infect Dis 2012; 205 (Suppl
2): S147-58. https://doi.org/10.1093/infdis/jir860.
2. World Health Organization
(WHO). Automated-real time nuclei acid amplification technology for rapid and
simultaÂneous detection of tuberculosis and rifampicin resistance: X-pert
MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary
tuberculosis in adults and children. WHO/ HTM/TB/2013.16 World Health
Organization https://apps.who.int/iris/handle/10665/112472
3. World Health Organization
(WHO). Using the Xpert MTB/ RIF assay to detect
pulmonary and extrapulmonary tuberÂculosis and
rifampicin resistance in adults and children. Expert Group
Meeting Report 2013. World Health OrgaÂnization.
https://apps.who.int/iris/handle/10665/112659
4. FIND [Internet]. GeneXpert negotiated prices. (date
unÂknown) (Cited 2020 June 23). En: https://www.finddx.org/pricing/genexpert/.
5. Hogan AB, Jewell BL, Sherrard-Smith D et al. Potential impact of the COVID-19
pandemic on HIV, tuberculosis, and malaria in low-income and middle-income
countries: a modelling study. Lancet Glob Health
2020; 8(9): e1132- e1141. https://doi.org/10.1016/S2214-109X(20)30288-6.
6. GLI Practical Guide to TB
Laboratory Strengthening, March 2017. En:
https://www.stoptb.org/wg/gli/assets/documents/GLI_practical_guide.pdf
7.
Programa Nacional de Control de la Tuberculosis. Normas TÃĐcnicas 2013, 4ta
EdiciÃģn Argentina.
https://bancos.salud.gob.ar/sites/default/files/2018-10/0000000278cnt-normas-tecnicas-2013-tuberculosis.pdf
8. World Health Organization.
Fluorescent light-emitÂting diode (LED) microscopy for diagnosis of tuberculoÂsis:
policy statement. World Health Organization 2011
WHO/HTM/TB/2011.8. En: https://apps.who.int/iris/handle/10665/44602
9.
OrganizaciÃģn Panamericana de la Salud 2008. GuÃa TÃĐcnica para el diagnÃģstico
bacteriolÃģgico de la Tuberculosis, Parte 2: Cultivo.
https://iris.paho.org/handle/10665.2/18616
10. World Health Organization. Xpert MTB/RIF impleÂmentation manual: technical and
operational âhow-toâ; practical considerations. World Health
Organization 2014. WHO/HTM/TB/2014.1 En:
https://apps.who.int/iris/handle/10665/112469
11. Eldholm
V, Monteserin J, Rieux A,
Lopez B, Sobkowiak B, Ritacco
V, Balloux F. Four decades of transmission of a
multidrug resistant Mycobacterium tuberculosis outÂbreak strain. Nat Commun 2015; 11-6: 7119. https://doi.org/10.1038/ncomms8119
12. Zifodya JS, Kreniske JS, Schiller
I et al. Xpert Ultra versus Xpert
MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with
presumptive pulmonary tubercuÂlosis. Cochrane Database of Systematic Reviews 2021, Issue 2. Art. No.:
CD009593. https://doi.org/10.1002/14651858.CD009593.pub5.
13. FIND. A multicentre
non-inferiority diagnostic accuracy study of the Ultra assay compared to the Xpert MTB/RIF assay. Version 1.8, February 2017. En:
https://www.finddx.org/wp-content/uploads/2019/12/Multicentre-noninferioriÂty-study-Ultra-Xpert-FEB2017-FINAL.pdf
14. World Health Organization.
Update on the use of nucleic acid amplification tests to detect TB and
drug-resistant TB: rapid communication. Geneva: World Health Organization;
2021.
https://www.who.int/publications/i/item/update-on-the-use-of-nucleic-acid-amplification-tests-to-detect-tb-and-drug-resistant-tb-rapid-communication.
15. WHO consolidated guidelines
on tuberculosis. Module 3: diagnosisârapid diagnostics
for tuberculosis detection. World Health Organization 2020.
https://apps.who.int/iris/bitstream/handle/10665/332862/9789240007307-eng.
pdf?sequence=1&isAllowed=y
16. Stop TB / USAID / GLI
Practical Guide to Implementation of Truenat Tests
for the Detection of TB and Rifampicin Resistance. Version 2: March 2021.
https://www.stoptb.org/assets/documents/resources/publications/sd/Truenat_Implementation_Guide.pdf.
17. World Health Organization The
use of loop-mediated isothermal amplification (TB-LAMP) for the diagnosis of
pulmonary tuberculosis: policy guidance. World Health
Organization 2016. WHO/HTM/TB/2016.11
https://www.who.int/publications/i/item/9789241511186
18. World Health Organization. The use of lateral flow urine lipoarabinomannan
assay (LF-LAM) for the diagnosis and screening of active tuberculosis in people
living with HIV. Policy guidance. World Health Organization 2015. WHO/
HTM/TB/2015.25. https://www.who.int/publications/i/item/9789241509633
19.
OrganizaciÃģn Panamericana de la Salud 2018. GuÃa TÃĐcnica para el diagnÃģstico
bacteriolÃģgico de la Tuberculosis, Parte 3: Pruebas de Sensibilidad. Programa
âFortalecimiento de la Red de Laboratorios de Tuberculosis en la RegiÃģn de las
AmÃĐricasâ - Lima: ORAS - CONHU; 2017).
https://www.paho.org/en/documents/technical-guide-bacteriological-diagnosis-tuberculosis-part-3-susceptibility-tests-2018
20. WHO consolidated guidelines
on tuberculosis. Module 3: diagnosis - rapid
diagnostics for tuberculosis detection, 2021 update. Geneva: World Health
Organization; 2021. https://www.who.int/publications/i/item/9789240030589
21. World Health Organization. The use of molecular Line Probe Assay for the detection of
resistance to second line anti-tuberculosis drugs. World
Health OrganizaÂtion 2016. WHO/HTM/TB/2016.07.
https://www.who.int/publications/i/item/9789241510561
22. World Health Organization.
The use of next-generation sequencing technologies for the detection of
mutations associated with drug resistance in Mycobacterium tuberÂculosis
complex: Technical guide. Geneva: World Health Organization 2018
(WHO/CDS/TB/2018.19). https://apps.who.int/iris/handle/10665/274443 Licence: CC
BY-NCSA 3.0 IGO.