Autor : Guillen Cuadros, Mayra1, Wittong Montesdeoca, Raquel1, Cornejo Lago, Silvia1,MacĂas Zambrano, Daniel2
1Hospital de Especialidades Portoviejo, Ministry of Health. 2 Hospital Instituto Ecuatoriano de Seguridad Social (IESS).
https://doi.org./10.56538/ramr.GWFC3776
Correspondencia : Dra. Mayra Guillen Cuadros Email: neumoguillen@hotmail.com Portoviejo, Ecuador
ABSTRACT
The group with compassionate use
of ruxolitinib for Covid-19 showed improved chest
images and a larger number of discharged patients, compared to group 1 (chloroÂquines and azithromycin), with a decrease in
inflammatory markers. There is one artiÂcle that described a case which
refractory to anti-IL6 therapy but responded to Jak-Stat
inhibition with ruxolitinib.1 The most
common comorbidity in both groups was arterial hypertension, followed by
diabetes type 2; group 1 showed a larger number of patients without comorbidities
(18 patients).
The number of male patients with
the disease caused by SARS-CoV2 was larger in group 1, with 31 males (62.0%),
compared to a total of 19 females (38.0%), whereas in group 2, 25.0% were
males, and 25.0% females. The severity of Covid-19 was defined as moderate: adolescent
or adult with clinical signs of pneumonia (fever, cough, dysÂpnea, tachypnea),
particularly SpO2 ≥
90% on ambient air; and severe: adolescent or adult with clinical signs of
pneumonia (fever, cough, dyspnea, tachypnea) plus some of the following:
respiratory rate > 30 breaths/min, severe respiratory distress or SpO2 < 90% on
ambient air.2
The acute respiratory distress
syndrome (ARDS) in both groups had an average ratio of pressure arterial oxygen
and fraction of inspired oxygen (PaFi) of 135.3 mmHg
in the ruxolitinib group versus 138.9 mmHg in the
control group.
Efficacy was defined as: decrease
in inflammatory markers, gasometric improvement in
the PaFi, lower oxygen requirement, lower number of
patients with severe symptoms admitted to the Intensive Care Unit, proof of the
drug’s safety 10 days after use, and detailed number of discharged patients.
Key words: Coronavirus Infection; Cytokines; Respiratory Distress Syndrome, Adult;
SARS-CoV2; Chloroquines
RESUMEN
El
uso compasivo de ruxolitinib en la covid-19 demostrĂł
una mejorĂa en las imágenes de tĂłrax y mayor nĂşmero de altas en el grupo que lo
usĂł vs. el grupo 1 (cloroquinas
y azitromicina), con descenso de los marcadores
inflamatorios. Existe un artĂculo que señalĂł que un caso que fue refractario a
la terapia anti-IL6, pero respondiĂł a la inhibiÂciĂłn de Jak-Stat
con ruxolitinib.1 La
comorbilidad más frecuente en ambos grupos fue la hipertensión arterial,
seguida por la diabetes tipo 2; el grupo 1 presentĂł un mayor nĂşmero de
pacientes que no presentaban comorbilidades (18 pacientes).
El
nĂşmero de hombres con enfermedad por SARS-CoV2 fue mayor en el grupo 1, con 31
hombres (62,0%) frente un total de 19 mujeres (38,0%), mientras que, en el
grupo 2, el 25,0% eran hombres y mujeres, el 25,0%. La gravedad de la covid-19
fue definida como moderada: adolescente o adulto con signos clĂnicos de
neumonĂa (fiebre, tos, disnea, taquipnea), en particular SpO2 ≥ 90% con aire ambiente; y grave:
adolescente o adulto con signos clĂnicos de neumonĂa (fiebre, tos, disnea,
taquipnea) más alguno de los siguientes: frecuencia respiratoria > 30
inspiraciones/min, dificultad respiratoria grave o SpO2 < 90% con aire ambiente.2
El
sĂndrome de dificultad respiratoria aguda (SDRA) en ambos grupos fue de un proÂmedio
de relaciĂłn entre la presiĂłn arterial de oxĂgeno y la fracciĂłn inspirada de oxĂÂgeno
(PaFi) en el grupo ruxolitinib
135,3 mmHg vs. Grupo control PaFi
138,9 mmHg.
Se
definiĂł la eficacia por descenso de los marcadores inflamatorios, mejorĂa
gasomĂ©Âtrica de la PaFi, menor requerimiento de
oxĂgeno, disminuciĂłn del ingreso a unidad de cuidados intensivos de los pacientes
con sintomatologĂa grave, demostraciĂłn de la seguridad del fármaco en los 10
dĂas posteriores a su uso y detallado del nĂşmero de casos con alta mĂ©dica.
Palabras
clave: Ruxolitinib; Infecciones por coronavirus; Citocinas; SĂndrome de Dificultad Respiratoria del Adulto;
SARS-CoV2; Cloroquinas
INTRODUCTION
The Covid-19 pandemic presented
approximately 10 874 146 million cases and around 521 355 deaths on a worldwide
level.3
In Ecuador, the rate was 147 033
cases confirmed by RT-PCR (reverse transcription-polymerase chain reaction),
with more than 10 800 deaths.4
The city of Guayaquil was one of
the most afÂfected areas, with the largest number of confirmed cases (17 973);
whereas in the ManabĂ province, 10 151 cases confirmed with swab PCR tests have
been reported up to now.5
The Intensive Care Units around
the country collapsed during the first four weeks. There was a group of
patients who couldn’t have access to a ventilator. The mortality rate was 7%.
The Hospital de Especialidades Portoviejo reÂceived during the first 6
months more than 12 000 cases of suspected Covid-19 based on clinical and
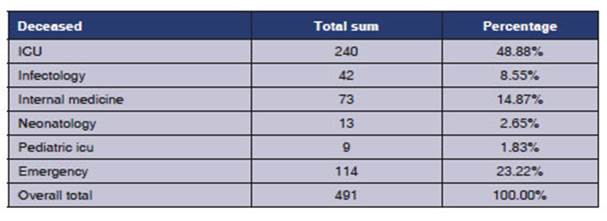
epidemiological criteria and chest imaging, with 491 deaths from SARS-CoV-2; and
the highest mortality rate occurred in the Intensive Care Unit (48.8%) (Table 1).
In view of the severity of this
disease, the Pulmonology and Infectology Service of
the HosÂpital de Especialidades Portoviejo, based on
poor pharmacological and therapeutic evidence about the Covid-19 pandemic,
created a protocol for the compassionate use of medication with ruxolitinib. It was used in SARS-CoV-2 cases with increased
inÂflammatory markers (Ldh [lactate dehydrogenase],
ferritin, D-dimer, IL6) and those with more than 50% of radiologic involvement
in chest images, at the IIB stage of the disease with hypoxemia. The hypothesis
included the fact that the use of ruxÂolitinib could
provide a benefit, because it reduces the cytokine levels, and so it could
reduce the number of patients admitted to the Critical Care Units with moderate
and severe acute respiratory distress syndrome; this would cause radiological
improvement and faster lymphocyte recovery.6
Secondary hemophagocytic
lymphohistiocyÂtosis (sHLH)
is a hyperinflammatory syndrome secondary to several
triggers, including sepsis, characterized by strong increase in cytokines with
multi-organ failure and a very high mortality rate.7
Ruxolitinib reduces the spleen volume and circulating levels of proinflammatory
interleuÂkins, particularly IL-6 and TNF-alpha.8
Recently, preliminary data from 7
patients with sHLH who were treated with ruxolitinib 15 mg (twice a day) showed promising results
regarding global surÂvival, and improvement has been observed in the
inflammatory markers such as ferritin and soluble IL-2 receptor.9
MATERIALS AND METHODS
Patients were studied from April
2020 to June 2020 at the Hospital de Especialidades
Portoviejo.
Inclusion criteria
Patients who met the following
criteria:
• High suspicion and diagnosis of
Covid-19.
• Chest X-ray and tomographies showing more than 50% of radiologic
involvement.
• PaFi ≤
250 mmHg.
• Rapidly worsening respiratory
failure requiring invasive ventilation.
• Increase in any of the systemic
inflammatory response inÂdicators: LDH 300 U/L, ferritin 1000 ng/mL, D-dimer 1500 ng/mL.
• SpO2 ≤ 93%.
Exclusion criteria
• Patients diagnosed with
Covid-19 plus chronic renal failure with clearance of less than 30 mL/h.
• Infectious diseases, such as
tuberculosis, HIV.
• Hypersensitivity to the active
principle.
• Pregnant women.
• Patients who weigh less than 50
kg.
• Platelets <50,000 cells/mmc.
• Hemoglobin < 8 g/dL.
• Neutrophils < 500 cells/mmc.
• Sepsis documented by pathogens
other than SARS-CoV2.
• Patients who didn’t sign
consent to the use of ruxolitinib.
DATA ANALYSIS
In the analysis of the information
gathered from the mediÂcal records of Covid patients,
we applied the quantitative paradigm through the use of descriptive statistics
and the creation of contingency tables, absolute and relative frequencies, and
the calculation of mean values, standard deviation and minimum and maximum
values. Inferential statistics is also applied to support the research
hypothesis through student-t techniques for independent samples and chi square
test, as applicable, depending on the type of variable. For data processing we
used the program Excel for windows and the statistical program SPSS, version
21.
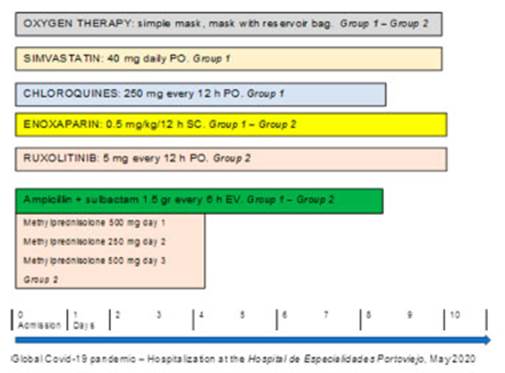
Treatment as compassionate use
Patients were randomly selected;
1:1 ratio. They were divided in two groups, 50 patients each, with a total of 100
patients diagnosed with Covid-19 through PCR: group 1 received 250 mg chloroquine phosphate every 12 h, for 7 days; group
2 received 5 mg ruxolitinib, twice a day, for 10
days. Also, systemic corticoids were used during 3 days, plus general measures
(Figure 1).
We performed daily
electrocardiogram control and susÂpended medication in patients with prolonged
QT interval ≥ 500 ms.
RESULTS
This study intends to compare the
use of two types of treatment in patients admitted to the Hospital Especialidades Portoviejo, Ecuador, an
institution in the province of ManabĂ with the sentinel surÂveillance system
for cases of SARS-CoV-2. Group 1 includes patients who receive chloroquine + azithromycin; and in group 2
patients receive ruxolitinib +
methylprednisolone.
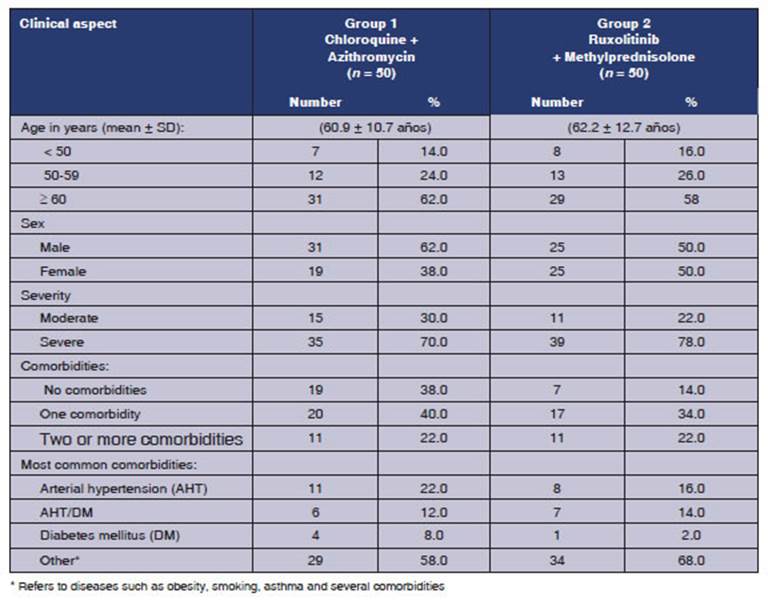
Table 2 shows the
clinical aspects of the cases according to the treatment group. One of the
variables is “age”, where patients aged 60 or older
predominate in both groups (62% in group 1 and 58% in group 2). Mean age was
60.9 ± 10.7 years and 62.2 ± 12.7 years, respectively.
Regarding sex, males
predominate in group 1 (62%), and account for 50% in group 2.
The degree of
severity that is most frequently presented is “severe” (70.0% in group 1 and
78.0% in group 2). With regard to comorbidities, the presence of an underlying
disease (40% in group 1 vs. 34% in group 2), or two or more associated diseases
(22% in both groups) predominate in both groups. The most frequently found
conditions were: hypertension (22% vs. 16%), hypertension and diabetes (12% vs.
14%), and diabetes (8% vs. 2%), group 1 vs. group 2, respectively.
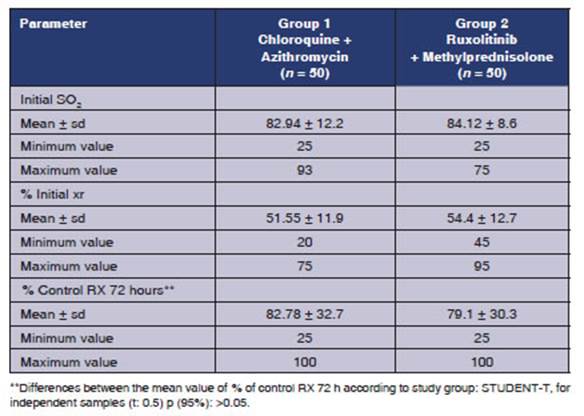
Table 3 shows some of
the clinical parameters used for the Covid diagnosis
protocol. SaO2 was measured upon admission,
and similar mean valÂues were observed in both groups (82.94 ± 12.2 in group 1,
and 84.12 ± 8.6 in group 2). Also, upon admission, we carried out chest X-ray
or lung tomography in patients who were able to move in order to observe the
percentage of involvement of the pulmonary parenchyma, and found 51.55 ± 11.9
in group 1, and 54.4 ± 12.7 in group 2. Then, 72% of
the patients underwent a control chest X-ray, and a lower percentage of
involvement was found in patients treated with ruxolitinib
+ methylprednisolone (79.1 ± 30.3 in group 2). An assay conducted in China
showed significant improvement in the chest computed tomographies
of 43 patients who received ruxolitinib.10
In order to show the differences
between the groups regarding the percentages of lung involveÂment through
imaging, we applied the student-t for independent samples. The results showed
that there weren’t any statistical differences between the groups in relation
to the percentage of lung involvement (p > 0.05; at 95% CI).
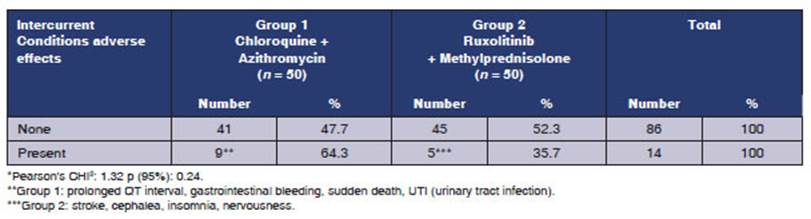
Adverse effects
Group 1: chloroquines + azithromycin: 4 out of 50 patients had prolonged QT interval, so
treatment with chloroquines was suspended. Those
patients, who were older than 70 years, died on days 7, 8 and 10 of
hospitalization. On day 4 of hospitalization, another patient showed lower
gastrointestinal bleeding; it is worth mentioning that the patient had arterial
hypertension, diabetes mellitus and nephrectomy due to clear cell cancer. Four
patients had sudden death on days 6 and 7 of hospitalizaÂtion.
Group 2: ruxolitinib + methylprednisolone: 1 out of 50 patients died on day 5 due to a stroke.
The other symptoms presented by 4 patients were mild, such as cephalea, insomnia and nervousness on days 1 to 3 of
hospitalization; we couldn’t deÂtermine if those symptoms were characteristic
of the ongoing disease.
The stroke could have been a
matter of causalÂity, considering the hypercoagulability syndrome of Covid-19.
The bibliography describes venous thrombosis in patients diagnosed with Sars- CoV-211, of 5%-15% in patients outside the
ICU, and up to 35% in patients inside the ICU.
Table 4 compares the adverse
effects found in patients treated with chloroquine +
azithromyÂcin (group 1) vs. those treated with ruxolitinib
+ methylprednisone (group 2). A higher number of
adverse effects and intercurrent conditions was
observed in group 1 (64.3%) compared to group 2 (35.7%). Some of the adverse
effects are: prolonged QT interval, gastrointestinal bleeding, sudden death,
UTI (group 1), and stroke, insomnia, nervousness (group 2). However, the
research hyÂpothesis about the differences between the groups according to the
treatment couldn’t be supported when applying the chi square, obtaining a p value
> 0.05, 95% CI.
Likewise, there are new lines of
research that would show with greater certainty the adverse efÂfects that could
be caused by the compassionate use of these treatments for this re-emerging
disease and its different variants.
It is necessary to mention there
was a limitaÂtion upon this research, since we couldn’t ask for the autopsy,
given the measures imposed by the Ministry of Public Health for the management
of corpses of patients diagnosed with Covid-19. Thus, it wasn’t possible to
determine the real cause of death of these patients, whose treatment (with its
adverse effects and complications) is showing effectivity
up to this day, and is being evaluated by specialists around the world.
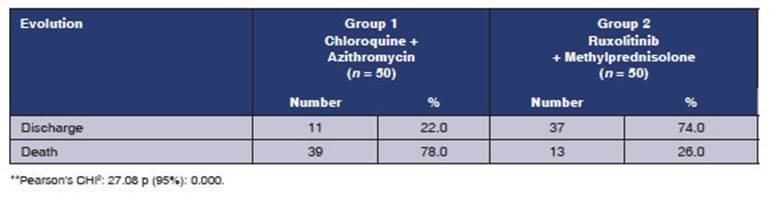
The number of patients referred
to the IntenÂsive Care Unit was higher in group 1: 30 patients. Only one of
those patients was discharged (3.3%), whereas 14 patients from group 2 were
referred to the ICU, and 4 of them recovered (28.5%).
CONCLUSION
We can’t attribute the decrease
in mortality and ICU admissions to the group using ruxolitinib,
since patients from this group received systemic corticosteroids; but, the increase
in the number of patients being discharged and the lower number of adverse
effects found in group 2 (ruxolitinib + methylprednisolone) are surprising. A significant increase
in mortality was seen with the use of chloroquines
and azithromycin.
This case report
invites health professionals to conduct studies with more statistical weight to
evaluate drugs that inhibit the cytokine storm induced by Covid-19.
Conflict of interest
Authors have no
conflict of interest to declare.
REFERENCES
1. Innes AJ, Cook LB,
Marks S, et al. Ruxolitinib para la inÂfecciĂłn
por COVID-19 grave resistente al tratamiento con tocilizumab. Br J Haematol. 2020;190:e181–e232.
https://doi.org/10.1111/bjh.16979
2.
OMS: OrganizaciĂłn Mundial de la Salud. [Internet]. Manejo clĂnico de la
COVID-19: Orientaciones provisionales. 2020;16. [citado 27 de Mayo
2020].Disponible en: https://apps.who.int/iris/bitstream/handle/10665/332638/WHO-
2019-nCoV-clinical-2020.5-spa.pdf
3. News.google
[Internet].Coronavirus covid19. Ecuador. 2020; [actualizado 8 septiembre
2020]. Disponible en: https://news.google.com/covid19/map?hl=es419&mid=/m/02j71&gl=US&ceid=US:es419
4.
Salud.gob.ec. [Internet].Covid-19 Ministerio de Salud Pública. Ecuador:2020;1-2. BoletinN°196. [Actualizado 12/09/20208].
Disponible en : https://www.salud.gob.ec/wp-content/uploads/2020/09/Boletin-196_Nacional_MSP.
pdf
5.
Salud.gob.ec. [Internet]. SituaciĂłn nacional por covid-19 infografĂa n°227.
Ecuador:2020; 1-5. [Inicio 29/02/2020 Corte
11/10/2020]. Disponible en :https://www.salud.gob.ec/wp-content/uploads/2020/10/INFOGRAFIA-NACIONAÂLCOVID19-COE-NACIONAL-08h00-11102020_new.pdf
6.
Cao Y, Wei J, Zou L, et al.
Ruxolitinib in treatment of seÂvere coronavirus disease 2019 (COVID-19): A
multicenter, single-blind, randomized controlled trial. J AllergyClin
Immunol. 2020;146:137-46.e.3.
https://doi.org/10.1016/j.jaci.2020.05.019
7.
Puja M, Daniel F, Michael B, et al. COVID-19: considere los sĂndromes de
tormenta de citocinas y la inmunosupresiĂłn. Lancet 2020;395.
8. Caocci
G, La Nasa G. Could ruxolitinib be effective in paÂtients with COVID-19 infection
at risk of acute respiratory distress syndrome (ARDS)? Ann Hematol. 2020;99:1675-6. .
https://doi.org/10.1007/s00277-020-04067-6
9.
Swamy Y, Paul S, Timothy B, et al.InhibiciĂłn
de la señalÂizaciĂłn de citocinas por ruxolitinib e implicaciones para el tratamiento con
COVID-19. Clin Immunol. 2020;218.
10. Cao Y, Wei J, Wang G, et al.
Reply. J Allergy Clin Immunol. 2020;146:1453-4.
https://doi.org/10.1016/j.jaci.2020.07.037
11.
Lopez Reyes, Oscullo G, Jimenez D, et al. Riesgo trombĂłtico
y Covid-19: revisiĂłn de la evidencia actual para un mejor enfoque diagnĂłstico y
terapéutico. Arch Bronconeumol.
2021;57:55-64.
https://doi.org/10.1016/j.arbres.2020.07.033