Autor Palmero Domingo1,2, Lagrutta Laura1,2, Aidar Omar1,2, Bartoletti Bruno1, Cruz VĂctor1, Gamberale Ana1, GarcĂa Ana1 , González Montaner Pablo1,2, Inwentarz Sandra2, Vescovo Marisa1,2
1Hospital de Infecciosas Dr. F. J. Muñiz, Ciudad AutĂłnoma de Buenos Aires, Argentina 2Instituto de TisioneumonologĂa Prof. Dr. RE. Vaccarezza, Ciudad AutĂłnoma de Buenos Aires, Argentina
Correspondencia : Domingo Palmero E-mail: djalmero@intramed.net
ABSTRACT
The emergence of resistant strains of Mycobacterium tuberculosis to multiple drugs and the difficulties of their diagnosis and treatment constitute a challenge to global public health. To face this challenge,
new anti-tuberculosis drugs, such
as bedaquiline, pretomanid,
and delamanid, as well as replacement drugs, such as fluoroquinolones, linezolid and clofazimine, are used. Based on
the evidence provided by multicenter
studies, drugs associated with a better prognosis of drug-resistant
tuberculosis have been discovered and, recently, a new classification has been proposed, as well as new totally oral regimens. In this review, we
describe current treatment regimens and practiÂcal pharmacological aspects required when prescribing
new drug-resistant tuberculosis treatment
regimens.
Key
words: Tuberculosis, Drug-resistance,
MDR-TB, Pharmacology
RESUMEN
La
emergencia de cepas resistentes de Mycobacterium
tuberculosis a múltiples droÂgas, las dificultades de su
diagnóstico y tratamiento constituyen un desafío a la salud
pública mundial. Para afrontar esta situación, se emplean nuevas
drogas antituberÂculosis, como bedaquilina,
pretomanid y delamanid,
así como drogas repropuestas, como fluoroquinolonas, linezolid y clofazimina. Con base en la evidencia brindada por estudios
multicéntricos, se han descubierto
fármacos asociados a un mejor pronóstico de la tuberculosis drogorresistente y, recientemente, se ha propuesto una
nueva clasifiÂcación, así como nuevos esquemas totalmente orales.
En esta revisión, describimos los esquemas de tratamiento actuales y los
aspectos farmacológicos prácticos necesarios a la hora de la
prescripción de los nuevos regímenes de tratamiento de la
tuberculosis drogorresistente.
Palabras
clave: Tuberculosis,
Drogorresistencia, TB-MDR, Farmacología
Recibido: 12/09/2021
Aceptado: 02/12/2022
INTRODUCTION
The global threat of drug-resistant tuberculosis (DR-TB) has promoted
the research of new treatÂment regimens, new drugs, and repurposed drugs1
(not originally
sold for TB, such as fluoroquinoÂlones, linezolid and clofazimine) together with the
traditionally called “second-line drugs” for the purpose
of improving the efficacy of treatment of these forms of the disease.
The objective of this review is
to briefly analyze current treatment regimens according to internaÂtional rules, and to describe dosages
in adults and children, mechanisms of action, adverse reactions, and use in cases of renal and liver failure, pregÂnancy and tuberculous
meningitis, of available drugs
to treat drug-resistant TB.
Thorough review on DR-TB found in2.
There are different DR-TB
categories3-5:
monoÂresistant TB, caused by strains of Mycobacterium
tuberculosis (Mtb) and resistant
to only one drug, the most
concerning being monoresistance to isoniazid (Hr) and rifampicin (Rr); multidrug-resistant TB
(MDR-TB), which shows resistance
to at least isoniazid (H)
and rifampicin (R); pre-extensively
drug resistant TB
(pre-XDR-TB), which is MDR
and also shows resistance
to one of the antituberculous fluoroquinolones
(levofloxacin or moxifloxacin); and at last, extensively resistant (XDR-TB), which apart from
being pre-XDR-TB, is also resistant to at least bedaquiline or linezolid (group
A of the World Health Organization, WHO).
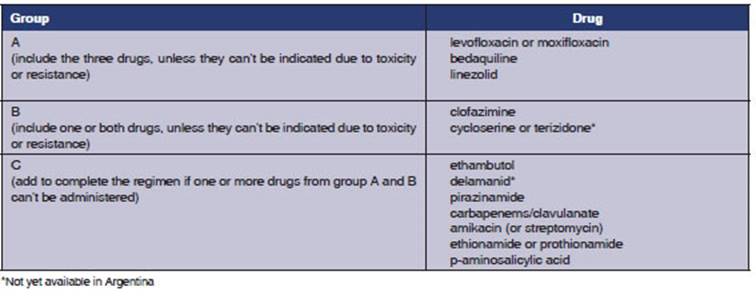
In
2018, the WHO published a
new classificaÂtion of drugs
to be used for DR-TB, updated in 20206 (Table 1) and based on the meta-analysis
of individual MDR-TB patients data published by Ahmad et al7.
Treatment regimens
of Hr-TB, Rr-TB and MDR-TB
The treatment recommended by the international guidelines for Hr-TB is a 6-month regimen with four
drugs, without initial phase: levofloxacin, pyrazinamide, rifampicin and ethambutol. TreatÂment duration is determined by
the need to comÂplete 6 months of levofloxacin6,
8.
Rr-TB is a category that arose
from the advent of the rapid
molecular method for
diagnosis called Xpert Mtb RIF, which detects in Mtb, with almost 100% specificity, the presence of the five most common
mutations of the RpoB gene that explain resistance to R9. Given
the fact that approximately 80% of the Rr strains
show additional resistance
to H10
and that a first-line
drug for the treatment of TB has been lost, Rr-TB
should be treated as MDR-TB6, 8.
According to new recommendations,
MDR-TB can be treated with
a 100% oral regimen including
the three drugs of the WHO Group A (bedaquiline, linezolid, fluoroquinolone), together with one
or two drugs
from Group B (cycloserine or clofaziÂmine). Bedaquiline is administered the first 6 months
(see Table
2), and the other three or four
drugs are given throughout the whole treatment, which lasts 18 months (it may
be shortened in mild
cases). Group C drugs would be left as replacement of Groups A and B if they can’t
be used due to resistance or adverse reactions6, 8.
Alternatives to isoniazid
and rifampicin for the treatment of DR-TB
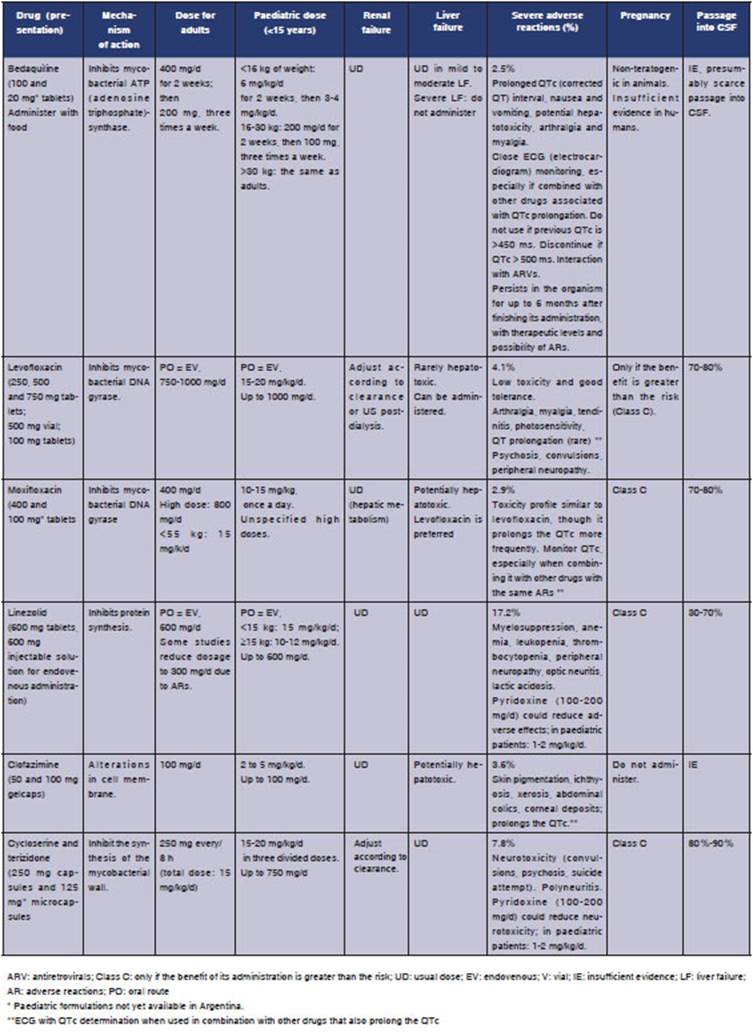
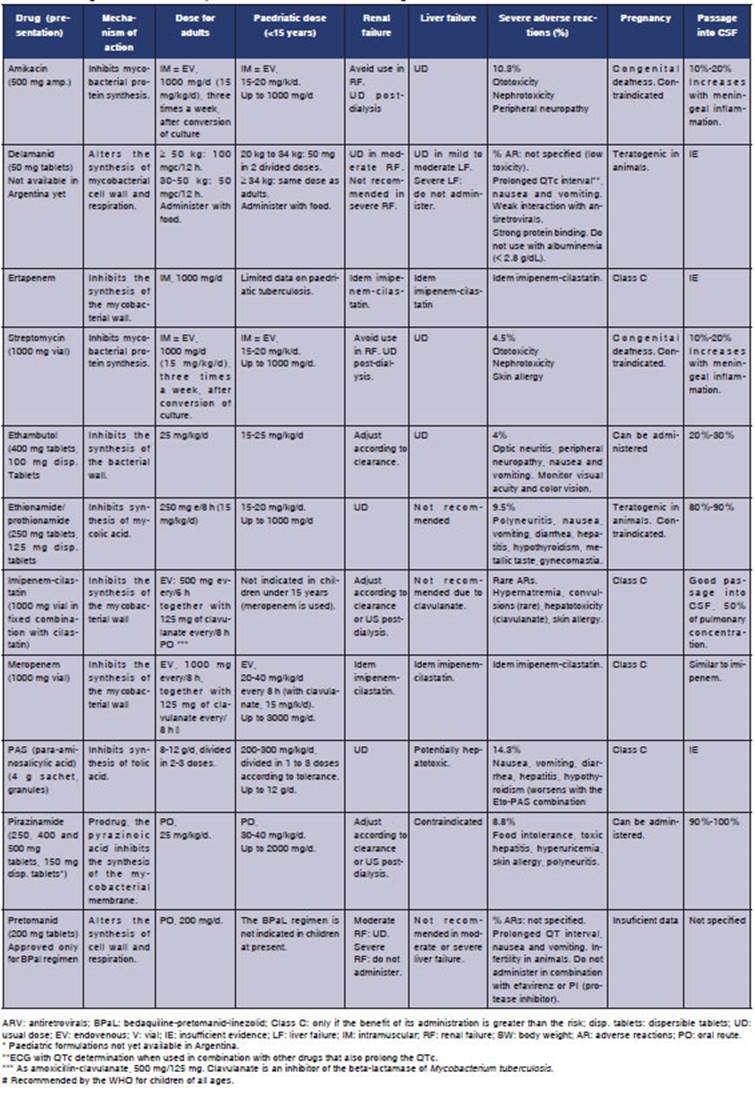
Tables 2 and 3 show every drug, mechanisms
of action, dosage in adults and children, most common adverse reactions, use in renal and liver
failure and pregnancy and passage into CSF (ceÂrebrospinal fluid), a fundamental element in
the treatment of tuberculous meningitis.
Treatment of XDR-TB11-13
As seen in its
definition, it implies resistance to at least H, R, one fluoroquinolone (levo or moxiÂfloxacin) and bedaquiline or linezolid. From
that minimum base of the definition (which already creÂates
a complicated situation but still leaves
other therapeutic options), resistance can be
extended practically to all
anti-TB drugs. Also, the antibioÂgram of multi-treated patients doesn’t correlate very well with
the clinical picture, as in MDR-TB, and it is more common to find differences between the phenotypic
and genotypic methods. So, it is important
to ask the patients detailed questions regarding their previous treatments and clinical and bacteriological responses. To sum up, the
design of a regimen for XDR-TB is individualized,
and no guidelines can be provided
as in other forms of DR-TB.
Regimens are indicated with drugs that
show persistent antibiogram
sensitivity plus those that weren’t previously
used, trying to get a minimum number
of potentially effective drugs (3 or 4). In an effort to improve
the diagnosis of these patients, the bedaquiline-delamanid
combiÂnation has been used, as well as bedaquiline alone, for one year
of treatment. New regimens such as BPaL (bedaquiline,
pretomanid and linezolid) will provide evidence
on the efficacy
of the new drug, pretomanid, under these circumstances14.
The prognosis of these patients is worse
than in other forms of DR-TB.
CONCLUSIONS
In this brief review of the practical
pharmacologiÂcal aspects of
drugs for the treatment of DR-TB in adults and children, we show drugs (such as bedaquiline, delamanid and pretomanid) that have been
specifically studied as antituberculosis drugs, something that hadn’t occurred since the discovery
of rifampicin, half a century ago. This is an auspicious
fact, together with the evidence
showing the activity of drugs that allow a 100% oral treatment in children and adults. There is
availability of regimens based on published
evidence for the treatment of monoresistant and multi-drug resistant TB. Unfortunately,
XDR-TB, the most severe mycobacterial resistance situation, is still a complex
problem in terms of therapeutic and prognostic aspects.
Conflict of interest
Authors declare there isn’t any
conflict of interest in relaÂtion to this publication.
REFERENCES
1. Rossato Silva D, Dalcolmo
M, Tiberi S, et al. New and repurposed
drugs to treat multidrug- and extensively drug-resistant tuberculosis. J Bras
Pneumol. 2018; 44: 153-60.
https://doi.org/10.1590/S1806-37562017000000436
2. Palmero DJ, Lagrutta L, Inwentarz SJ, Vescovo M, Aidar OJ, González Montaner PJ. Tratamiento de la
tuberculosis drogorresistente en adultos y
niños. Revisión narrativa. Medicina (Buenos Aires). 2021. E-pub:
https://www.meÂdicinabuenosaires.com/adelantos/
3. Organización Mundial de la Salud (OMS). Definiciones y
marco de trabajo para la notificación de Tuberculosis-revisión
2013 (actualizado en diciembre de 2014). WHO/ HTM/TB/2013.2. ISBN 978 92 4
350534 3.
4. World Health
Organization (WHO). Meeting report
of the WHO expert consultation on the definition of extensively drug-resistant
tuberculosis, 27-29 October 2020. Geneva: World Health Organization;
2021. CC BY-NC-SA 3.0 IGO. En:
https://www.who.int/publications/i/item/meeting-report-of-the-who-expert-consultation-on-the-definition-of-extensively-drug-resistant-tuberculosis
5. Roelens M, Migliori
GB, Rozanova L, et al. Evidence-based
Definition for Extensively Drug-resistant
Tuberculosis. Am J Respir Crit
Care Med. 2021; 204):
713-22. https://doi.org/10.1164/rccm.202009-3527OC.
6. WHO. Consolidated guidelines
on tuberculosis. ModÂule 4: treatment
- drug-resistant tuberculosis treatÂment.
Geneva: World Health Organization; 2020. CC BY-NC-SA 3.0 IGO. En:
https://www.who.int/publications/i/item/9789240007048
7. Ahmad N, Ahuja SD, Akkerman OW, et al. Collaborative
Group for the Meta-Analysis of Individual Patient Data in MDR-TB treatment–2017.
Treatment correlates of successful outcomes in pulmonary multidrug-resistant
tuberculosis: an individual patient
data meta-analysis. Lancet.
2018; 392: 821-34. https://doi.org/10.1016/S0140-6736(18)31644-1
8. Nahid P, Mase SR, Migliori
GB, et al. Treatment of Drug-
Resistant Tuberculosis. An Official ATS/CDC/ERS/IDSA Clinical
Practice Guideline. Am J Respir Crit Care
Med. 2019; 200: e93-e142. https://doi.org/10.1164/rccm.201909-1874ST.
9. WHO. WHO consolidated guidelines on tuberculosis.
Module 3: diagnosis – rapid diagnostics
for tuberculosis detection.
2020. CC BY-NC-SA 3.0 IGO. En:
https://www.who.int/publications/i/item/9789240029415
10. WHO. Global tuberculosis report
2021. Geneva: World Health Organization; 2021. CC BY-NC-SA 3.0 IGO. En:
https://www.who.int/publications/i/item/9789240037021
11. Hewison C, Bastard
M, Khachatryan N, et al. Is
6 months of bedaquiline enough? Results from the compassionÂ
ate use of bedaquiline in Armenia
and Georgia. Int J Tub Lung Dis. 2018;22:766-72.
https://doi.org/10.5588/ijtld.17.0840
12. Conradie F, Diacon
AH, Ngubane N, et al. Nix-TB
Trial Team. Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. N Engl J Med. 2020; 382: 893-902.
https://doi.org/10.1056/NEJMoa1901814
13. Pecora F, Dal
Canto G, Veronese P, Esposito S. Treatment
of Multidrug-Resistant and Extensively
Drug-Resistant Tuberculosis in Children:
The Role of Bedaquiline and
Delamanid. Microorganisms.
2021; 9: 1074. https://doi.org/10.3390/microorganisms9051074
14. Conradie F, Everitt
D, Olugbosi M, et al. High rate
of successful outcomes treating highly resistant TB in the ZeNix study of pretomanid, bedaquiline and alternative doses and durations
of linezolid. Abstract
OALB01LB02. 11th IAS Conference on
HIV Science Abstract Supplement JIAS 2021;24(S4):e25755-Page
70 En: https://onlinelibrary.wiley.com/doi/epdf/10.1002/jia2.25755; consultado
octubre 2021.
15. Sentinel project.
Management of Drug-Resistant Tuberculosis in Children: A Field Guide. Boston,
USA: The Sentinel Project for Pediatric Drug-Resistant
Tuberculosis; November 2018, Fourth
edition. En:
http://sentinel-project.org/2019/04/10/sentinel-field-guide/; consultado
octubre 2021.
16. Dheda K, Gumbo
T, Maartens G, et al. The epidemiology, pathogenesis, transmission, diagnosis, and management
of multidrug-resistant, extensively
drug-resistant, and incurÂable tuberculosis. Lancet Respir Med.
2017; S2213-2600: 30079-6. v https://doi.org/10.1016/S2213-2600(17)30079-6
17. WHO. Companion Handbook
to the WHO Guidelines for the Programmatic
Management of Drug-Resistant TuberÂculosis. Geneva: World Health Organization;
2014. WHO/ HTM/TB/2014.11. En:
https://apps.who.int/iris/bitstream/handle/10665/130918/9789241548809_eng.pdf;
consultado octubre 2021.
18. Lange C, Dheda
K, Chesov D, Mandalakas AM,
Udwadia Z, Horsburgh CR Jr.
Management of drug-resistant tuberÂculosis. Lancet. 2019; 394: 953-66. https://doi.org/10.1016/S0140-6736(19)31882-3.
19. Huynh J, Marais
BJ. Multidrug-resistant tuberculosis infection and disease in children: a review of new and reÂpurposed drugs. Ther Adv Infect
Dis. 2019; 6: 1-16. https://doi.org/10.1177/2049936119864737.
20. WHO. Rapid communication on updated guidance
on the management
of tuberculosis in children and adolescents.
Geneva: World Health Organization; 2021. Licence: CC
BY-NC-SA 3.0 IGO. En: https://www.who.int/publications/i/item/9789240033450
21. Seddon JA, Wilkinson
R, van Crevel R, et al. Knowledge
gaps and research priorities
in tuberculous meningitis. Wellcome
Open Res. 2019; 4: 188. https://doi.org/10.12688/wellcomeopenres.15573.1
22. Wilkinson RJ, Rohlwink
U, Misra UK, et al. Tuberculous
Meningitis International Research Consortium:
TubercuÂlous meningitis. Nat
Rev Neurol. 2017;
13: 581-98. https://doi.org/10.1038/nrneurol.2017.120.
23. Palmero D, González Montaner P, Cufré
M, García A, VescoÂvo M, Poggi
S. First series of patients
with XDR and pre- XDR TB treated
with regimens that included meropenen-clavulanate
in Argentina. Arch Bronconeumol. 2015; 51: e49-52. https://doi.org/10.1016/j.arbres.2015.03.012
24. Tiberi S, D’Ambrosio
L, De Lorenzo S, et al. Ertapenem in the treatment of multidrug-resistant tuberculosis: first
clinical experience. Eur Respir J. 2016; 47: 333-6.
https://doi.org/10.1183/13993003.01278-201