Autor : PĂ©rez Conde Lucas1
1Pulmonary Laboratory IADT Instituto Argentino de DiagnĂłstico y Tratamiento, Buenos Aires, Argentina.
Correspondencia : DE-mail: lucasperezconde@yahoo.com.ar
ABSTRACT
The prevalence of respiratory
complications subsequent to COVID-19 pneumonia is currently unknown, but the
data obtained from previous coronavirus outbreaks may provide important
information. The preliminary evidence supports the hypothesis that some
survivors could develop long-term respiratory sequelae, being the pulmonary
fibrosis the most important.
We report three cases of patients
hospitalized in the ward with moderate to severe COÂVID-19, never requiring
mechanical respiratory assistance (MRA). Follow-up computÂed tomography scans
after discharge showed images compatible with post-pneumonia pulmonary
fibrosis.
Key words: COVID-19. Pulmonary fibrosis. Sequelae
Accepted: 09/11/2021
INTRODUCTION
The prevalence of respiratory
complications after COVID-19 pneumonia is currently unknown, but the data
obtained from previous coronavirus outbreaks may provide important information1.
Some reports state that between
20% and 60% of survivors of the global SARS (severe acute respiÂratory
syndrome) outbreak caused by SARS-CoV (SARS-associated coronavirus) and
MERS-CoV (Middle East respiratory syndrome coronavirus) experienced some
persistent physiological deteÂrioration and pulmonary images compatible with
fibrosis1.
The preliminary evidence supports
the hypothÂesis that some survivors could develop long-term respiratory
sequelae. Pulmonary fibrotic anomaÂlies have been detected three weeks after
the onset of symptoms, regardless of the degree of severity of the disease
(mild, moderate or severe)2.
We present three patients with
moderate to severe pneumonia, who required oxygen, antibiotÂics and
corticosteroids but never needed invasive mechanical respiratory assistance
(MRA). Follow-up computed tomography scans between 30 and 60 days after
discharge showed images with interÂstitial infiltrates compatible with
post-COVID-19 pneumonia pulmonary fibrosis.
CLINICAL CASES
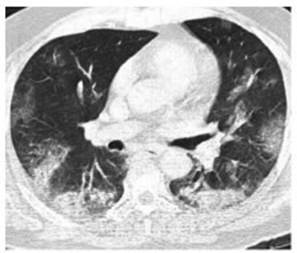
Case 1: 84-year-old female
patient, with history of light smoking (5 packages per year), obesity, arterial
hypertenÂsion (AHT), chronic renal failure and coronary disease. The patient
was hospitalized in the ward due to moderate COVID-19 pneumonia (according to
the severity criteria of the 2007 ATS/IDSA [American Thoracic
Society/Infectious Diseases Society of America] Guidelines) (3) for 34 days;
chest CT done on admission (Figure 1). She received antiÂbiotic treatment
(ampicillin/sulbactam [AMS], 1.5 g every 6 h for 10 days, and clarithromycin,
500 mg every 12 h for 10 days), oxygen therapy (with nasal cannula, between 3
L/min and 4 L/min for 3 days), and corticosteroid therapy (dexaÂmethasone, 8 mg
per day for 10 days). Follow-up CT scan done two months after the onset of
symptoms (Figure 2).
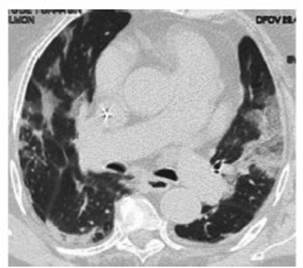
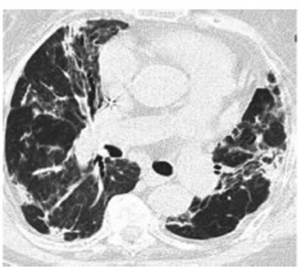
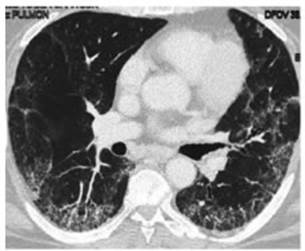
Case 2: 58-year-old female
patient with history of obesity, a professional nurse. Hospitalized due to
moderate to severe COVID-19 pneumonia (according to the severity criteria of
the 2007 ATS/IDSA Guidelines)3 for 18 days, requiring oxygen therapy
(with nasal cannula at 6 L/min for 6 days) and non-invasive ventilation (NIV),
(pressure support ventilation [PSV], inspiratory positive airway pressure
[IPAP]: 10, expiratory positive airway pressure [EPAP]: 5 for 2 days) in a
closed unit; she also received 2 units of convalescent plasma, antibiotic
treatment (AMS, 1.5 g every 6 h for 10 days and clarithromycin, 500 mg every 12
h for 10 days) and corticosteroid therapy (dexamethasone, 8 mg per day for 10
days). Chest CT done on admission (Figure 3). Follow-up CT scan done 2 months
after discharge due to persistent dyspnea FC II/III. (Figure 4).
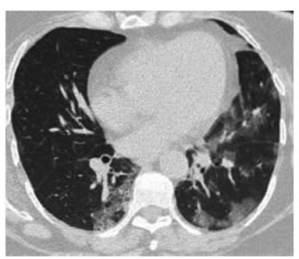
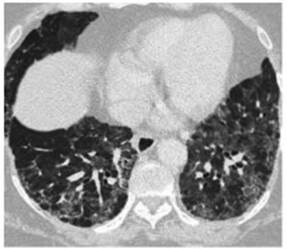
Case 3: 63-year-old male patient
with history of smokÂing (32 packages per year), diabetic. Hospitalized in the
ward due to moderate COVID-19 pneumonia (according to the severity criteria of
the 2007 ATS/IDSA Guidelines) (3) for 17 days. While hospitalized, the patient
required oxygen therapy (with nasal cannula at 3 L/min for 2 days),
corticosteroid therapy (dexamethasone, 8 mg per day for 10 days) and antibiotic
treatment (AMS, 1.5 g every 6 h for 7 days and clarithromycin, 500 mg every 12
h for 7 days). Chest CT done on admission (Figure 5). 20 days after disÂcharge,
the patient attended the on-call service with dysÂpnea, functional class
III/IV. CT was done with pulmonary thromboembolism (PTE) protocol, without
positive result, and progression of septal thickening to subpleural predomiÂnance
and honeycombing were evidenced in parenchymal window (not present in the
previous study). Symptoms are interpreted as secondary to the sequelae of
previous pneuÂmonia; no new supplementary tests were done (Figure 6).
DISCUSSION
It’s a well-known fact that many
patients suffering from acute respiratory distress syndrome (ARDS) experience
deterioration of their quality of life, years after the disease, despite the
breakthrough in clinical care related to the pulmonary protection strategies of
mechanical ventilation.
A percentage of ARDS survivors
develop a fibroproliferative response characterized by the accumulation of
fibroblasts and deposit of collagen and other elements of the extracellular
matrix in the lung.
The development of severe
fibroproliferative lung disease has been associated with bad progÂnosis and
high mortality rates4.
Four stages of COVID-19 at chest
CT have been described: early
stage (0 to 5 days after the onset of symptoms), characterized by
normal findings or mainly ground glass opacities; progressive stage (5-8 days after the onset of
symptoms), may show increase in ground glass opacities and crazy paving;
peak stage (9
to 13 days after the onset of symptoms), characterized by progressive
consolidation; and late
stage (≥ 14 days after the onset of symptoms),
characterized by a gradual decrease in consolidation and ground glass opaciÂties,
whereas signs of pulmonary fibrosis can start manifesting (including
interstitial parenchymal bands, lung architectural distortion and traction
bronchiectasis)5.
Patients referred to chest CT
must do it withÂout contrast, unless CT pulmonary angiogram is required to
detect pulmonary thromboembolism (PTE)5.
If a follow-up CT scan is to be
done, we suggest the use of a low radiation dose protocol in order to minimize
the radiation load5.
A cohort study of COVID-19
patients, with follow-up done six months after discharge, which, as mentioned
by the authors, is the largÂest study with the longest follow-up duration of
discharged patients, showed that the evaluation of the lung function in a
considerable proporÂtion (22%-56% in different degrees of severity) of
participants showed certain deterioration of the diffusing capacity of the
lungs for carbon monoxide (DLCO), six months after the onset of symptoms. This
was consistent with the findings that the abnormal patterns most freÂquently
found in the chest CT were interstitial pulmonary infiltrates (ground glass
infiltrates and septal thickening).
The respiratory viral infection
could potenÂtially induce a different fibroblast activation in the
convalescence phase. We found that, the more severe the disease in the acute
phase, the more important the alteration in the DLCO and tomographic pattern.
The results of this study didn’t
suggest that corticosteroids can accelerate pulmonary lesion recovery in the
evaluation of the lung function and chest images, even though the evidence has
shown the benefits of this treatment for patients with severe COVID-19 in the
acute phase6.
In agreement with these results,
another study was published with the follow-up of patients who required
hospitalization in the intensive care unit (ICU) and were evaluated three
months after hospital discharge. The follow-up included symptoms and quality of
life, anxiety and depression questionnaires, lung function tests, 6-minute walk
test (6MWT) and chest CT. We found that there is a relationship between age and
days of MRA and tomographic findings. The main patterns that were found were
ground glass infiltrates (59.6%), septal thickening (80.7%) and bronchiectasis
(71.9%). The rate of reticular and fibrotic lesions was 49.1%, even higher than
the rate of survivors of other viral types of pneumonia, including SARS, H1N1
and H7N97.
Recent studies have also shown
that patients with COVID-19 are more frequently hospitalized, have longer
hospital stays and higher risk of deÂveloping SDRA, in comparison with patients
with other acute respiratory diseases7, 8.
In relation to the large number
of patients with pneumonia caused by SARS-CoV2 and the possible risk of pulmonary
sequelae, it is imporÂtant to do a follow-up in order to detect possible
complications.
To do so, various societies of
respiratory medicine have published some recommendaÂtions for the clinical and
radiological follow-up that suggest control of pulmonary images and lung
function tests mainly according to the seÂverity of the condition and the
presence at the moment of clinical symptoms, at a reasonable interval1, 9, 10.
The purpose of this series is to
show examples of possible sequelae in patients who have suffered COVID-19
pneumonia.
Conflict of interest
The author declares no conflicts
of interest.
Acknowledgment
To doctors Darío
Raúl Rey and Carlos Gustavo Di Bartolo, for their contributions
REFERENCES
1. George PM, Barratt SL,
Condliffe R, et al. RespiraÂtory follow-up of patients with COVID-19 pneumonia.
Thorax 2020; 75: 1009-16. http://dx.doi.org/10.1136/thoÂraxjnl-2020-215314
2. Raghu G, Wilson KC. COVID-19
interstitial pneumoÂnia: monitoring the clinical course in survivors. Lancet Resp 2020; 8: 839-42. https://doi.org/10.1016/S2213-2600(20)30349-0
3. Mandell LA, Wunderink RG,
Anzueto A, et al; InfecÂtious Diseases Society of America; American Thoracic
Society. Infectious Diseases Society of America/American Thoracic Society
consensus guidelines on the manageÂment of community-acquired pneumonia in
adults. Clin Infect Dis. 2007; 44(Suppl 2):S27-72.
https://doi.org/10.1086/511159
4. Burnham EL, Janssen WJ, Riches
DWG, et al The fibroproliferative response in acute respiratoÂry distress
syndrome: mechanisms and clinical sigÂnificance. Eur Respir J 2014;43:276-85.
https://doi.org/10.1183/09031936.00196412
5. Kwee TC, Kwee RM. What the
Radiologist Needs to Know. Radiographics 2020; 40: 1848-65. https://doi.org/10.1148/rg.2020200159
6. Huang C, Huang L, Wang Y, et
al. 6-month consequences of COVID-19 in patients discharge from hospital: a
cohort study. Lancet 2021; 397: 220-32.
https://doi.org/10.1016/S0140-6736(20)32656-8
7. González J, Benítez ID, Carmona P, et
al. CIBEREÂSUCICOVID Project (COV20/00110, ISCIII). PulmoÂnary
Function and Radiologic Features in Survivors of Critical COVID-19: A 3-Month
Prospective CohortÂChest. 2021;160: 187-98.
https://doi.org/10.1016/j.chest.2021.02.062
8. Shah SJ, Barish PN, Prasad PA, et al. Clinical feaÂtures, diagnostics, and outcomes of patients presenting
with acute respiratory illness: A retrospective cohort study of patients with
and without COVID-19. ECliniÂcalMedicine 2020; 27: 100518.
https://doi.org/10.1016/j. eclinm.2020.100518
9. Sibilaa O, Molina-Molina M, Valenzuela C, et al.
Documento de consenso de la Sociedad Espanola de Neumología y
Cirugía Torácica (SEPAR) para el seguimiento clínico
post-COVID-19. Op Resp Arch 2 2020;278-83. https://doi.org/10.1016/j.opresp.2020.09.002
10. National Institute for Health
and Care Excellence, PracÂtitioners RC of G, Scotland HI. COVID19 rapid
guideline: managing the long-term effects of COVID-19. NICE Guidel. 2020; 1-35.