Autor : BalcÃĄzar Torres Jonathan1,OssÃĐs Juan Manuel1, CalderÃģn Juan1, Vicente Luis1, Nazzo Viviana1, Carrasco Gladys1, Ahumada RosalÃa1, IbÃĄÃąez Teresa1, Favaloro Roberto1, Candiotti Mariano1, Bertolotti Alejandro1, CÃĄneva Jorge Osvaldo1
1Hospital Universitario FundaciÃģn Favaloro, CABA, Argentina
Correspondencia : Jonathan BalcÃĄzar Torres: jtorres@ffavaloro.org; drjmbt2012@gmail.com
Abstract
The infectious complication is the most common condition after a
transplantation. There is a limited description regarding the prevaÂlence of
donor-associated infections (DAIs) in lung transplant (LTx) recipients. There
are reports of DAIs in LTx recipients of 7.6%, with documented prophylactic
failure of 5.6%.
Objective: to estimate the frequency of donor-associated
infections after lung transplantation and their outcome in terms of overall
survival (OS).
Methodology: an observational, descriptive study, carried
out in a transplant center in Argentina between
January 2018 and June 2020. The study included all the patients who underwent a
transplantation within such period and those with defined/proven DAIs.
Results: during the aforementioned period, 65 LTx were
performed in 64 individuals (one patient underwent transplantation and
subsequent retransplantation in the same study period). The median age was 39
(12-72) years. Cystic fibrosis was the main reason for transplantation (26.2%)
In 61/65 cases (94%), germs were isolated from biological samples collected
from the donor: 78.6% in the preservation liquid, 73.7% in donor secretions,
21.3% surgical samples, and 4.9% blood cultures. Donor-associated infections
were identified in 2/61 cases (prevalence of 3.1%; 95% CI: 0.4-10.7%), with a
median posttransplant OS of 12 months, and an OS of 98.4% (95% CI: 91.7-99.9%).
Conclusion: the prevalence of DAIs in LTx recipients in the
present series was 3.1%: higher than the figures documented for solid organ
transplants in general (< 1%), but lower than the numbers found in the few
published reports (7.6%).
Key words: Infection; Donor; Transplant; Lung
Received: 4/13/2021
Accepted: 10/14/2021
Introduction
Lung transplantation (LTx) has become an accepted treatment option
for end-stage pulmonary paÂrenchymal and vascular diseases. The donorâs
pretransplant screening is very important and should be conducted rigorously in
order to minimize as much as possible the risk of transmission of certain
infectious processes.
This study has the objective of estimating the frequency of
donor-associated infections after LTx and their outcome in terms of overall
survival. Complications occur frequently and may lead to medium or long-term
graft dysfunction and a decrease in survival. According to the registry of the
International Society for Heart and Lung Transplantation (ISHLT), LTx 1-, 2-
and 5-year survival rates are 80%, 65%, and 53%, respectively1.
Donor-derived disease transmissions are defined as any disease present
in the organ donor that is transÂmitted to at least one of the recipients1. Bacteria or fungi can be
transferred to the donor graft through contamination during recovery,
preservation or manipulation, or during the transplantation. Infectious complications
are a common cause of morbidity and mortality, and the most important cause of
death during the first year. More than two thirds of those conditions affect
the respiratory tract1.
The prognosis for LTx recipients has considerably improved in recent years,
thanks to the thorough selection of donors and recipients, and better surgical
techniques, postoperative care and graft preservation methods.
This work addresses specific aspects of DAIs, which are one of the
most important problems that need to be handled during the first days after
LTx. So, more studies are necessary in order to answer questions about DAIs in
LTx recipients.
Materials
and Methods
Study
Design
This is an observational, descriptive, prevalence study. It was
designed in accordance with the guidelines of the Strengthening the Reporting
of Observational Studies in Epidemiology (STROBE) declaration. It was carried
out in patients who underwent a LTx at the Hospital Universitario
Fundación Favaloro (HUFF) between January 2018 and June 2020.
Population
and sample
Inclusion criteria. Patients who underwent a LTx basing
on the ISHLT standards, according to the prioritization criteria for the
allocation of organs for transplant of the Unique Central National Institute
Coordinator of Ablation and Implant (INCUCAI, for its acronym in Spanish),
regardless of the age group, condition or type.
Transmission events reported in this review refer to
proven/defined cases in compliance with the definitions of imputability for
donor origin of disease transmission (according to USA and Europe literature)2. A DAI is considered as
proven (according to the American criteria) whenever there is clear evidence of
the same infectious disease in the donor and at least one of the recipients,
and all the following conditions must be fulfilled: suspected transmission
event, laboratory evidence of suspected organism (or malignancy) in a
recipient, laboratory evidence of the same organism (or malignancy) in other
recipients (if there are several recipients), laboratory evidence of the same
organism or maligÂnancy in the donor, and if there is pretransplant laboratory
evidence, it shall indicate that the same recipient tested negative for this
organism before the transplant. A DAI is considered as defined or true
(according to the European criteria) when there is conclusive evidence beyond
reasonable doubt for attributing the disease to the process or the transplanted
organ2.
Exclusion criteria. This review didnât take into
account cases in which subsequent clinical follow-up wasnât possible.
Data collection. Data were collected in an
encrypted, online Microsoft Excel 365 electronic spreadÂsheet.
Statistical
analysis
Technical considerations. A p-value < 0.05 was considered
statistically significant. The statistical analysis was conducted with the R
v.3.6.3 program (R Foundation for Statistical Computing; Vienna, Austria).
Sample size calculation. Considering a 95% confidence
interval (CI), a 5% margin of error, and a general DAI rate between transplants
of 1% (Theodoropoulos N & Ison M, Transplant Infections, 2016), we
estimated a required sample of 16 cases.
Descriptive statistics. Numeric variables were described as
mean (standard deviation) or median (interquartile range, IQR), according to
their statistical distribution (Kolmogórov-Smirnov test). DeÂscriptive
variables were described in frequencies (percentages), with their respective
confidence interval (95% CI), if applicable.
Ethical
considerations
The research protocol was approved by the HUFF Ethics Committee.
All the patients signed the corresponding informed consent for attendance
purposes. Data custody was guaranteed at all times pursuant to Personal Data
Protection Law No. 25.326 (Ministry of Justice and Human Rights, ArgenÂtine Republic).
This research was conducted in accordance with the Nuremberg Code of 1947, and
the Declaration of Helsinki of 1964 and subsequent amendments (last version,
2013).
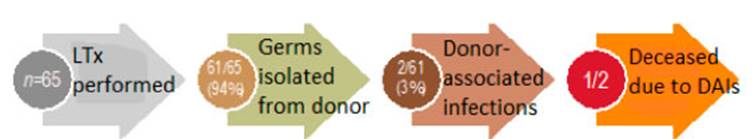
Results
A total of 65 LTx were performed in 64 individuals in our hospital
between January 2018 and June 2020 (one patient underwent a transplantation and
subsequent retransplantation within the same study period). All the individuals
were followed up until the end of the study period. The median age was 39 years
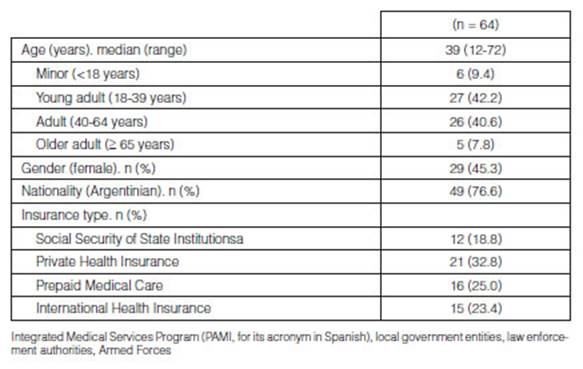
(12-72); 29/64 were females (45.3%). Table 1 summarizes the
sociodemographic characteristics of the patients included in the study.
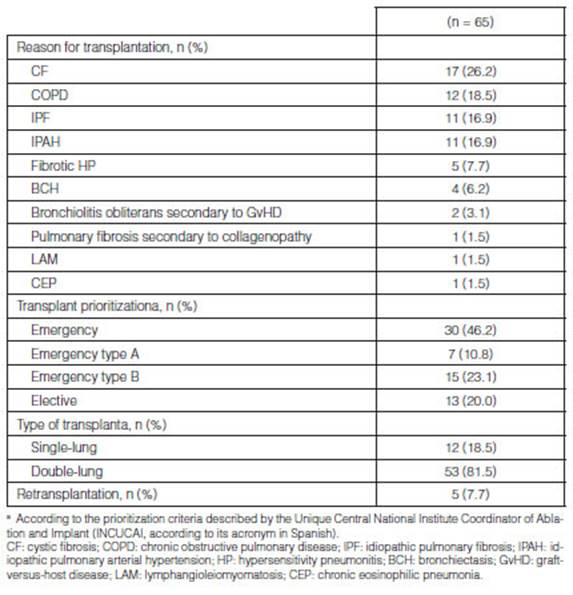
Cystic fibrosis (CF) was the main reason for performing a LTx
(17/65; 26.2%), followed by chronic obstructive pulmonary disease (COPD)
(12/65; 18.5%), idiopathic pulmonary fibrosis (IPF) (11/65; 16.9%) and
idiopathic pulmonary arterial hypertension (IPAH) (11/65; 16.9%). 30/65 cases
(46.2%) met the criteria for an emergency transplant. 53/65 cases (81.5%)
underwent a double-lung transplantaÂtion. 5/65 cases (7.7%) underwent a
retransplantation, taking into account the fact that within this study period
there was one case in which the patient underwent a transplantation and a subsequent
retransplantation at the end of the study. (Table 2).
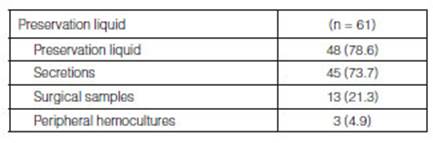
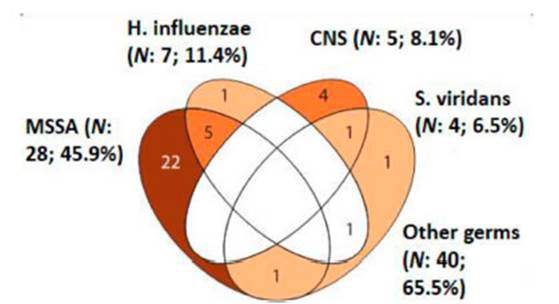
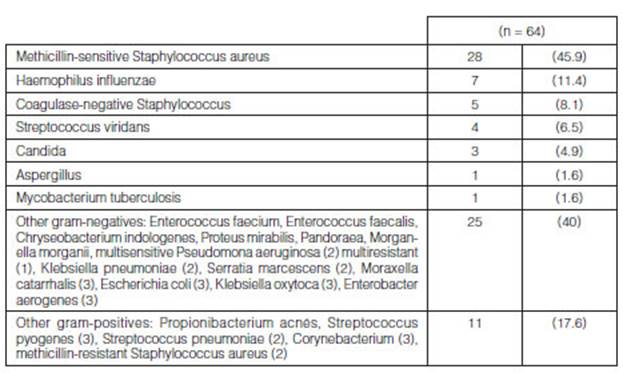
From all the LTx that were carried out (n=65), at least one germ was
isolated in 61/65 (94%) bioÂlogical samples collected from the donor (Table
3). The main germs isolated from these samples were methicillin-sensitive
Staphylococcus aureus (MSSA) (28/61; 45.9%), followed by Haemophilus influenzae
(HE) (7/61; 11.4%), coagulase-negative staphylococcus (CoNS) (5/61; 8.1%), and
Streptococcus viridans (4/61; 6.5%); other germs (40/61; 65.5%) (Figure 1
and Table 4).
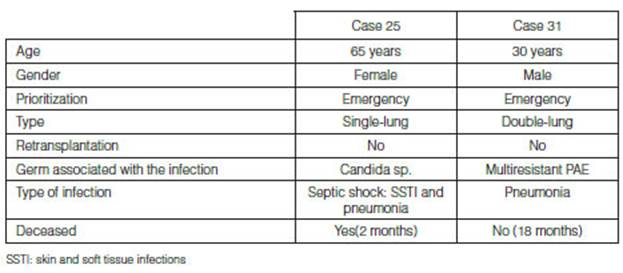
Donor-associated infections were identified in 2/61 (3%) cases
which are detailed in Table 5. Both cases received targeted treatment
according to the sensitivity of the rescue medication, and a frequency of 3.1%
(95% CI 0.4-10.7%) and a 12-month posttransplant median overall survival
(interquartile range, IQR 6-23) were identified in the total number of
transplants. One patient died from a DAI, after develÂoping septic shock
secondary to a skin and soft tissue infection of the surgical wound and
pneumonia with isolation of candida sp., sensitive to amphotericin B,
vorinocazole, caspofungin and anidulafungin. The patient did not respond to
treatment with azoles with susceptibility testing and adequate plasma
concentrations of the drugs in use. (Figure 2). It is surprising that
the two cases that showed donor-associated infection met the prioritization
criteria for an emergency transplant.
Posttransplant infections are jointly one of the most common and
most severe complications of a transÂplantation.(3)
The purpose of the patientâs screening is the identification of active and
latent infections that might pose a risk for the recipient and include:
clinical examination, epidemiologic inquiry and lab tests3.
The tests used to detect infectious diseases are those recommended
for every organ donor by the Organ Procurement and Transplantation Network
(OPTN) and for organ recipients4.
Unexpected disease transmissions are defined as the transmission
of a pathogen from the donor to the recipient, despite donor selection to
discard the presence of an infection. They can occur due to the donorâs
incomplete or inexact information, or failure of communication or the system,
if the donor has acquired the infection recently and is still in the eclipse
period or the serologic window period, or if the donor is infected with a rare
or emergent pathogen not included in standard detection protocols. Unexpected
transmissions are more likely to occur in the context of a deceased donor;
however, they may also occur in the transplant of a living donor2.
The course of posttransplant infections is divided in three
periods related to the risk of infection with specific pathogens: First
month after transplantation: could be caused by preexistent infection of
the donor or recipient and infectious complications of the transplantation
surgery and hospitalization. The main effects of exogenous immunosuppression
are still not clear.(5) 1 to 6 months after transÂplantation:
there is usually the maximum effect of immunosuppression and the patients
have higher risk of developing opportunistic infections. However, there may be
residual problems of the periopÂerative period. Prophylaxis delays but doesnât
eliminate the risk of infections that may occur months after prophylaxis is
over5. More than 6 to
12 months after transplantation: most patients receive stable and reduced
levels of immunosuppression. These patients are subject to community-acquired
pneumonias caused by respiratory viruses, pneumococcus, Legionella or other
common pathogens. The âlate cytomegalovirus (CMV)â might occur in patients who
received prophylaxis during the first three to six months5.
Epidemiology
of posttransplant infections
The rate of bacterial infections in lung and heart transplants
(mainly respiratory infections) is much Donor-Associated Infections in Lung
Transplant Recipients
higher than that observed in other recipients of solid organ
transplants (SOTR).6 Transmission of bacteria through
the graft is very common in LTx, showing bronchial colonization even in 50% of
the cases. However, it is very rare in other SOTRs in whom the transplanted
organ is usually sterile7.
Basing on available data from the USA and France, donor-derived
diseases are transmitted in less than 1% of transplants in general4. The mortality rate as a
consequence of DAIs in transplants in genÂeral, was 22%2.
The incidence of DAIs during the first 2 postoperative weeks has decreased
markedly because of antibiotic prophylaxis; most bacterial pneumonias occur in
the intermediate (< 6 months) and late postoperative period (> 6 months).
The overall cumulative incidence during the first year after transplantation is
∼70% and it remains
high beyond the first year (30%-40%). Nearly three-quarters of all bacterial
pneumonias are caused by Pseudomonas species and Enterobacteriaceae, and the
remainÂder primarily by Staphylococcus aureus, Enterococcus species and
Hemophilus influenzae8, 9.
In our series, the main isolated germs were methicillin-sensitive
Staphylococcus aureus, 45.9%; Haemophilus influenzae, 11.4%, and
coagulase-negative Staphylococcus, 8.1%.
The second most common infectious complication after LTx is CMV
disease. The reported incidence without prophylaxis in larger series ranges
between 53% and 75%6. In
our transplantation program, prophylaxis for high-risk patients includes
treatment with valganciclovir for no less than 6 months and sequential controls
with plasma PCR (polymerase chain reaction) for the detection of CMV.
Invasive infections with Candida species occur during the first
postoperative month, and most of them are transmitted through the donated
organ. The most common presentations are candidemia, necrotizing bronchial
anastomotic infection, mediastinitis and infection and disruption of aortic
anasÂtomosis after heart-lung transplantation8,
10. In our series, one of the two patients who developed a
DAI died due to a septic shock secondary to a skin and soft tissue infection of
the surgical wound and pneumonia caused by candida sp. with no response to
treatment with azoles with susceptibility testÂing and adequate plasma
concentrations. Heavy growth of Candida species in the donor bronchus is a
significant obstacle for accepting the organs for transplantation. The sequelae
are mediastinitis, sepÂsis, or involvement of the great vessels leading to
mycotic aneurysms and consecutive rupture. In one series, 3 of 4 lung
transplant recipients with heavy growth of Candida species developed
mediastinitis, which was uniformly fatal11.
There are different modes of transmission of infection by
Mycobacterium tuberculosis in this populaÂtion3:
as reactivation of previous infection, primoinfection, exogenous reinfection
and infection transÂmitted by the transplanted organ. In approximately 6% of
LTx recipients, the mean posttransplant interval in which Mycobacterium
tuberculosis is detected is 115 days. In 40% of the cases, the diagnosis was
obtained from explanted lungs6.
In our series, we isolated a tuberculous granuloma: one of the donor grafts had
an indurated lesion in the right upper lobe. Intraoperative exeresis was
performed with subsequent bacteriological and anatomo-pathological isolation.
In subsequent controls through fibrobronchoscopy and cultures, there wasnât any
evidence of disease development in the recipient. In general, Mycobacterium
tuberculosis screening isnât performed in deceased donors, but should be done
in all living donors4, 12.
Despite the immunosuppression, we observed and adequate response to
antituberculous treatment and low incidence of adverse secondary effects13. Active tuberculosis
(TB) in a donor is a contraindication to donation; if a deceased donor is
believed to have tuberculosis, his/ her organs shall not be used unless active
TB infection can be definitively discarded4,
12, 14.
Diagnostic
considerations
As part of our lung transplant program institutional protocol, a
patient who is undergoing immediate postoperative clinical or radiological
signs of infection receives a fibrobronchoscopic examination with
bronchoalveolar lavage (BAL) and, in cases of transbronchial biopsy (TBB), the
diagnostic yield is alÂmost 70%15;
it also allows for the inspection of the airways, which may reveal anastomotic
problems or tracheobronchial aspergillosis. The bacteriological examination of
bronchial lavages of the donor lung is a prerequisite for the treatment of
subsequent invasive infection in transplant recipients, even the growth of
normal oral flora in the donor is considered to be a risk factor for early
bacterial pneumonia in the recipient.
The BAL is very sensitive to most pathogens, the TBB is the only
means to diagnose acute rejection and pneumonitis caused by CMV16;
its sensitivity and specificity is almost 100%. Computed tomography can be
useful for the differential diagnosis of bilateral infiltrative lung diseases
and detect bronchial, mediastinal or vascular complications17.
Most centers now perform routine surveillance bronchoscopies after
lung transplantation. Besides the early detection of asymptomatic episodes of
significant acute rejection or CMV pneumonitis in ∼20% -30% of procedures, it allows
the early identification of cases colonized by Aspergillus.8
Review of
previous works that document donor-associated infections in LTx recipients
There is little literature about cases of DAI in lung transplant
recipients. In this review, there were 3 studies that showed statistics which
allowed us to compare our experience. Ruiz I et al.18 evaluated recipients who
survived more than 24 hours and their respective donors. The global incidence
of donor infections was 52% (103 out of 197 donors). The types of
donor-associated infections were contamination isolated in preservation fluids
(n = 30, 29.1%), graft colonization (n = 65, 63.1%) and bacteremia (n = 8,
7.8%). Donor infection rates werenât statistically different between patients
who received mechaniÂcal ventilation for 48 hours and those who received less
or more than 48 hours. There were bacterial or mycotic DAIs in 15 LTx (7.6%).
In this experience, 25% of donors with bacteremia and 14.1% of colonized grafts
were responsible for the transmission of the infection. Two patients died from
a DAI. Microorganisms for which it is very difficult to design effective
prophylactic regimens that caused inÂfection were Aspergillus fumigatus,
Stenotrophomonas maltophilia and MSSA. Excluding these cases, failure of
prophylaxis occurred in 5.6% of procedures.
Low et al19 reported that in 28 out of 29
bronchial lavages from donors grew at least one microorganÂism. Microorganisms
most frequently identified were Staphylococcus spp. and Enterobacter spp. In
43% of the cases, similar microorganisms were isolated from the recipient
tracheobronchial tree, 21% of which had a DAI. Waller et al20 made a retrospective comparison
of the result of 123 donors in 125 consecutive lung or heart transplants with
technical success. The microbial contamination of the routine bronchial lavage
of the donor was nearly 60%. Five lung transplant recipients died because of a
DAI.
Characteristics
of antibiotic treatment
There arenât any guidelines or standardized regimens regarding the
choice of the perioperative antibiotic therapy. Antibiotic prophylaxis in LTx recipients
shall be initiated with broad-spectrum antimicrobial agents in order to cover
gram-negatives and gram-positives.
We recommend that antibiotic coverage in lung transplant
recipients should be initiated with a broad-spectrum agent and modified on the
basis of cultures obtained from the donor19;
in our transplantation program we use vancomycin and ciprofloxacin for
non-colonized recipients with non-septic diseases who hadnât been hospitalized
during the last month, except for patients with septic lung disease (cysÂtic
fibrosis or bronchiectasis) who must receive antimicrobial agents adapted to
their pretransplant cultures for at least 2 weeks4;
all of these guidelines subsequently adjusted to the isolates obtained. In one
series, this approach reduced the incidence of early postoperative bacterial
pneumonia from 33% in a historical control group to 13% (p = 0.005)11.
In our program, according to the recommendation of the experts, we
indicate nebulized tobramycin or colistin once the patient arrives at the ICU
after surgery as prophylaxis in recipients that show previous gram-negative
colonization. The duration of the prophylaxis depends on the results of the
cultures of respiratory samples obtained from the donor and recipient at the
moment of the LTx. If the cultures are negative, the prophylactic antibiotic
agents are removed from the third to the fifth day; if they are positive, or in
cases of recipients with septic pulmonary disease, the antibiotic treatment is
adjusted and maintained for 2 weeks or until the cultures are negative.
With this approach, whenever a clinically significant
microorganism is isolated in a respiratory sample within the first 3 months, a
specific intravenous antibiotic therapy is initiated, even if the patient is
asymptomatic. The only situations in which the treatment shall not be initiated
are colonization by oral streptococcus or CoNS6.
Conclusion
Donor-derived diseases are increasingly being recognized as causes
of morbidity and mortality that occur usually during the early posttransplant
period. Bacterial infection is the most common infectious complication in LTx
recipients. Of all the lung transplantations performed in our series (n= 65),
at least one germ was isolated in 61/65 (94%) biological samples collected from
the donor. The main species isolated from donor cultures were
methicillin-sensitive Staphylococcus aureus, Haemophilus influenzae and
coagulase-negative Staphylococcus, mainly in preservation liquid, 78.6%; donor
secretions, 73.7%; and only 3 cases showed bacteremia in the donor (4.9%). The
rate of bacterial infections in lung and heart transplants (mainly respiratory
infections) is much higher than that observed in other SOTR.
The prevalence of DAIs in LTx recipients in our series was 3.1%,
higher than the figures documented in solid organ transplants in general (<
1%) but lower than the numbers found in the few published reports about LTx
(7.6%).(18) One of the 2 cases with identified DAI
died after developing septic shock secondary to pneumonia and a skin and soft
tissue infection of the surgical wound with isolation of candida, with no
response to treatment with azoles.
A high OS was observed; thus, there was low mortality associated
with the LTx. It is possible that the use of prophylactic measures when
selecting the suitability of the donor pulmonary graft and of antibiotic
prophylaxis guidelines in recipients has a strong impact on such OS.
Recommendations
Regarding the management of the pulmonary graft, we recommend the
following routine:
Send a sample of the preservation solution in which the organ was
received for culture. Request culture results and take appropriate measures
regarding the recipients. The possibility of a DAI shall be considered in all
early infections and patients with atypical clinical courses.
The DAI is a common event after lung transplantation with fatal
consequences that could be avoided with an adequate prophylactic antibiotic
regimen that has to be modified according to the type of miÂcroorganisms
isolated from the cultures of samples obtained from donors, grafts,
preservation fluids and recipients.
References
1. Ison MG, Nalesnik MA. An Update on Donor-Derived Disease
Transmission in Organ Transplantation. Am J Transplant. 2011; 11(6): 1123-30.
2. White SL, Rawlinson W, Boan P, et al. Infectious Disease
Transmission in Solid Organ Transplantation: Donor Evaluation, Recipient Risk,
and Outcomes of Transmission. Transplant Direct [Internet]. 20 de diciembre de 2018;
5(1). Disponible en: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6324914/
3. Evaluación infectólogica.
https://www.incucai.gov.ar/files/docs-incucai/Materiales/profesionales/14-evaluacion_infectologica.pdf.
4. Theodoropoulos N, Ison MG. Donor-Derived Infections: Incidence,
Prevention, and Management. En: Ljungman P, SnydÂman D, Boeckh M, editores.
Transplant Infections [Internet]. Cham: Springer International Publishing;
2016. p. 113-27. Disponible en: http://link.springer.com/10.1007/978-3-319-28797-3_8
5. Fishman JA. Infection in the solid organ transplant recipient.Updated Aug 2020.
6. Len O, Roman A, Gavaldà J. Risks and Epidemiology of
Infections After Lung or HeartâLung Transplantation. En: LjungÂman P, Snydman
D, Boeckh M, editores. Transplant Infections [Internet]. Cham: Springer
International Publishing; 2016. p. 167-83. Disponible en:
http://link.springer.com/10.1007/978-3-319-28797-3_11
7. Yuste JR, Pozo JL del, Quetglás EG, Azanza JR.
Infecciones más comunes en el paciente trasplantado. An Sist Sanit Navar
[Internet]. agosto de 2006 [citado 21 de septiembre de 2020];29. Disponible en:
http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S1137-66272006000400016&lng=en&nrm=iso&tlng=en
8. Speich R, van der Bij W. Epidemiology and Management of
Infections after Lung Transplantation. Clin Infect Dis. 2001; 33(s1): S58-65.
9. Dauber JH, Paradis IL, Dummer JS. Infectious complications in
pulmonary allograft recipients. Clin Chest Med. 1990; 11(2): 291-308.
10. Palmer SM, Alexander BD, Sanders LL, et al. Significance of
blood stream infection after lung transplantation: analysis in 176 consecutive
patients. Transplantation. 2000; 69(11): 2360-6.
11. Zenati M, Dowling RD, Dummer JS, Paradis IL, Arena VC,
Armitage JM, et al. Influence of the donor lung on development of early
infections in lung transplant recipients. J Heart Transplant. octubre de
1990;9(5):502-8; discussion 508-509.
12. Subramanian AK, Morris MI, AST Infectious Diseases Community
of Practice. Mycobacterium tuberculosis infections in solid organ
transplantation. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg.
2013; 13 Suppl 4: 68-76.
13. Bravo C, Roldán J, Roman A, et al. Tuberculosis in lung
transplant recipients. Transplantation. 2005; 79(1): 59-64.
14. Morris MI, Daly JS, Blumberg E, et al. Diagnosis and
management of tuberculosis in transplant donors: a donor-derived infections
consensus conference report. Am J Transplant Off J Am Soc Transplant Am Soc
Transpl Surg. 2012; 12(9): 2288- 300.
15. Chan CC, Abi-Saleh WJ, Arroliga AC, et al. Diagnostic yield
and therapeutic impact of flexible bronchoscopy in lung transplant recipients.
J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 1996; 15(2):
196-205.
16. Boehler A, Vogt P, Zollinger A, Weder W, Speich R. Prospective
study of the value of transbronchial lung biopsy after lung transplantation.
Eur Respir J. 1996; 9(4): 658-62.
17. Soyer P, Devine N, Frachon I, et al. Computed tomography of
complications of lung transplantation. Eur Radiol. 1997; 7(6): 847-53.
18. Ruiz I, Gavaldà J, Monforte V, et al. Donor-to-host
transmission of bacterial and fungal infections in lung transplantation. Am J
Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2006; 6(1): 178-82.
19. Low DE, Kaiser LR, Haydock DA, Trulock E, Cooper JD. The donor
lung: infectious and pathologic factors affecting outcome in lung
transplantation. J Thorac Cardiovasc Surg. octubre de 1993; 106(4): 614-21.
20. Waller DA, Thompson AM, Wrightson WN, et al. Does the mode of
donor death influence the early outcome of lung transÂplantation? A review of
lung transplantation from donors involved in major trauma. J Heart Lung
Transplant Off Publ Int Soc Heart Transplant. 1995; 14(2): 318-21.