Autor : Muñoz-Lombo Jenny Patricia1,2, Garrido-Valencia Guillermo Alberto1,3 Pacheco Robinson2
1 Universidad Libre, Faculty of Health Sciences, Internal Medicine, Cali, Colombia 2 Universidad Libre, Research Group on Epidemiology and Services, GRIEPIS, Cali, Colombia 3Department of Pulmonology and Sleep Lab, AIREC Specialized Medical Group, Cali, Colombia
Correspondencia :Jenny Patricia Muñoz Lombo. Calle 11A # 70-35. Conjunto Residencial Santa Paula, apartment 804, tower 4. Neighborhood: Capri.Cali. Colombia. Zip code: 760033. E-mail: ideasenproceso@hotmail.com
Abstract
Introduction: Obstructive sleep apnea (OSA) is a common entity present in 9% to 38%, but with an important underreporting of 85%1. Classically, it is observed in middle-aged, obese and sleepy men2, closely related to multiple comorbidities of cardiovascular3-6, respiratory7 and metabolic8, 9 and is associated with increased mortality. Recent studies indicate that majority of people with type 2 diabetes also has OSA. The aim of this study was to assess the prevalence and severity of OSA and risk factors contributing to it among people with chronic and severe type 2 diabetes. Methods A total of 203 people with type 2 diabetes (mean age: 54 ± 8 years, 145 males, 58 females, HbA1c ≥ 7% [53mmol/mol] types, generating high costs of health systems.10 The purpose of this study was to determine the frequency and identify the clinical factors associated with obstructive sleep apnea within the adult population of a specialized center in Cali, Colombia.
Materials and Methods: Observational, analytical case-control study nested within a cohort. First-time polysomnography records were analyzed in adults with clinical suspicion of OSA in a specialized center. The characteristics of the study population were summarized through descriptive statistics. Associations were determined through OR, 95% CI and values of p ≤ 0.05 taken as significant for the statistical tests. Through multivariate logistic regression, a 6-variable model was identified, where the 6 variables independently explain the event.
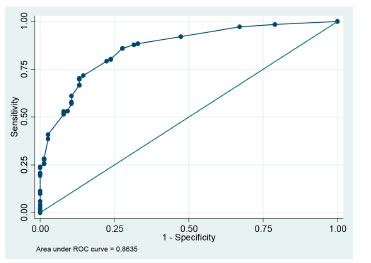
Results: We analyzed 566 polysomnographies, where the prevalence of OSA was 85.3% (483 of 566, 95% CI 82.4-88.35%), the mean age was 51.80 ± 13.73 years and 50% were men The final model included male gender (OR 4.46 95% CI 2.04-8.04, p <0.000), hypertension (OR 3.78 95% CI 2.48-8.04, p <0.000), Mallampati grade IV (OR 4.14, 95% CI 2.41-7.10, p <0.000) and excessive sleepiness (OR 5.70 95% CI 1.66-19.53, p <0.006) and normal weight (OR 0.48 95% CI 0.24-0.97, p <0.043).
Conclusion: The predictive probability showed that being male, hypertensive with Mallampati grade IV and excessive sleepiness are associated independently with OSA, while normal weight decreased the risk.
Key words: Obstructive sleep apnea; Polysomnography; Associated factors.
Introduction
Obstructive sleep apnea (OSA) is an entity that belongs to the group of breathing disturbances during sleep11, and is the product of recurrent upper airway collapse induced by sleep, which reduces (hypopnea) or stops (apnea) the airflow to the respiratory system; this causes hypoxemia and hypercapnia that excite the cortical area of the nervous system resulting in an activation of the pharyngeal muscles, thus recovering the permeability of the airway12. According to the American Academy of Sleep Medicine, the following conditions are necessary for an OSA diagnosis: signs and symptoms (associated somnolence, fatigue, insomnia, snoring or observed apnea) or associated medical or psychiatric disorders (that is to say, hypertension, coronary heart disease, atrial fibrillation, congestive heart failure, stroke, diabetes, cognitive dysfunction or mood disorder) together with five or more respiratory events mostly obstructive per hour of sleep during the polysomnography. A 15/h frequency of obstructive respiratory events meets the criteria, even in the absence of symptoms or associated disorders13. The classification of severity was made basing on the frequency of said events into: mild (AHI: 5-15 events/hour), moderate (AHI: 16-30 events/hour) and severe (AHI > 30 events/hour)14.
The prevalence of OSA in the general population oscillates between 9% and 38%, increasing with age, reporting a prevalence of up to 84% within the geriatric population1. However, the disease is not widely suspected, thus a > 85% underreporting is estimated15, 16.
A close relationship has been shown between the OSA and multiple comorbidities of the cardiovascular type, such as myocardial infarction, need for coronary revascularization, development of heart failure and cardiovascular death in 2.5 times3. The risk of suffering a stroke increases 6% for every increase in AHI4, and the atrial fibrillation increases three times in comparison with the general population5. In a hypertensive population, it may affect up to 40%, even 90% in cases of resistant hypertension6. This indicates that for every increase in the AHI the risk of developing resistant hypertension increases 1%, whereas for every 10% decrease in overnight oxygen saturation, the risk of developing OSA increases 13%17.
With regard to the respiratory disease, the OSA may increase mortality in patients with chronic obstructive pulmonary disease up to 1.79 times7 and show even double the postoperative cardiopulmonary complications18 with a stronger probability to desaturate and being transferred to the intensive care unit after the surgery19.
The OSA is also associated with metabolic disorders. A prospective evaluation performed in a diabetes treatment center in India found that 23.65% of diabetic patients had OSA (AHI ≥ 15), with a higher percentage of diabetic complications such as cardiovascular disease, retinopathy and neuropathy8. The Sleep Heart Health Study showed that this entity affects mainly men, in whom a 10 kg weight gain increases five times the risk of elevating the AHI > 159; it is also reported that 45% of patients who underwent bariatric surgery have OSA, obtaining after the procedure a three times reduction of their AHI, thus improving minimum oxygen saturation, sleep efficiency and the latency of rapid eye movement sleep (REM sleep)2.
A study carried out in Italy estimates that medical costs generated by the treatment of comorbidities of patients with OSA add up to €2.9 trillion, representing 55% of total medical costs in Italy, 6% of which belong to the diagnosis and treatment of OSA, and 49% due to lack of diagnosis and prevention of related comorbidities. So, timely detection of OSA would save the governments €1.5 trillion10. Other costs derived from OSA are related to low productivity, days lost from work and work accidents20 and car crashes caused by microsleep21.
Given the high prevalence, the high underreporting, the close relationship with frequent comorbidities and limited information available in Colombia about this topic, this research has the purpose of determining the frequency and identifying the clinical factors associated with the obstructive sleep apnea-hypopnea syndrome within the adult population in a specialized center in Cali, Colombia.
Materials and Methods
Design
We performed an observational, case-control study nested in a cohort of patients treated at a center that specializes in pulmonology, immunology, cardiology and cardiac and lung rehabilitation who showed clinical suspicion of OSA and had been referred by specialized physicians for diagnosis confirmation. Data collection was carried out in a retrospective manner.
Study Population
We analyzed the records of adult patients of 18 years or older who went for the first time to AIREC specialized medical group, in the city of Cali, Colombia, due to clinical suspicion of OSA to be confirmed by diagnostic polysomnography type I performed with the Philips Respironics Alice-6 polysomnograph. Records showed at least 4 hours of sleep (TST > 240 min). We excluded the records of previous polysomnographies of pregnant women, patients with craniofacial deformities, tumors in the upper airway, restrictive lung disease, history of tuberculosis, incomplete clinical information limiting the performance of the corresponding analyses or evidence of previous OSA treatment with continuous positive airway pressure (CPAP).
Every record with AHI ≥ 5 event/hour determined through polysomnography, positive for OSA was considered as case, and every record with AHI values of less than 5 measured during the same period from January 2014 until September 2016 was defined as control.
Statistical Analysis
In order to sum up the characteristics of the study population we used descriptive statistics. Quantitative variables were presented through their measures of central tendency and of dispersion. In order to compare data distribution, we used the Kolmogórov-Smirnov Test and considered the values of p ≤ 0.05 as significant. Qualitative variables were summed up as proportions and were presented in frequency tables. Prevalence was estimated as a proportion, taking the number of OSA positive cases as numerator and all patients with suspicion of OSA as denominator. Also, the stratified general prevalence of the event was estimated according to severity.
In order to explore possible associations between exposure variables and result variables (having or not having OSA), we used the odds ratio (OR) as a measure of association, with its respective confidence intervals of 95% (95% CI). The statistical significance of associations between numerical variables was calculated with the Mann Whitney U Test, and for dichotomous variables we conducted the Chi2 or Fischer Test as applicable. We considered the values of p ≤ 0.05 as significant to reject null hypotheses.
In order to adjust possible confounding factors and determine the weight of each variable in the presence of the obstructive sleep apnea-hypopnea syndrome, a binary logistic regression was used. When building the models, we included the variables that showed a statistical significance of ≤0.20 in the bivariate analysis. Modelling was made through the Backward strategy and the most parsimonious model was presented through the Hosmer-Lemeshow Test. For the degree of success of the model we used the ROC (receiver operating characteristic) curve. All the analyses were carried out with software Stata® (Stata Corp, 2011, Stata 12 Base Reference Manual, College Station, TX, USA).
Ethical Considerations
This study has been approved as minimal risk research by the research bioethics committee of the Universidad Libre and the institutional committee, having expressed there isn’t any kind of conflict of interest.
Results
Between January 2014 and September 2016, 1089 subjects with suspicion of OSA were evaluated in the institution through polysomnography. The subjects had been referred by the following medical specialties: internal medicine, family medicine, otorhinolaryngology, neurology, psychiatry, pulmonology, cardiology and rheumatology. After applying the selection criteria, 566 records were analyzed in this study. Exclusion criteria are shown in Figure 1.
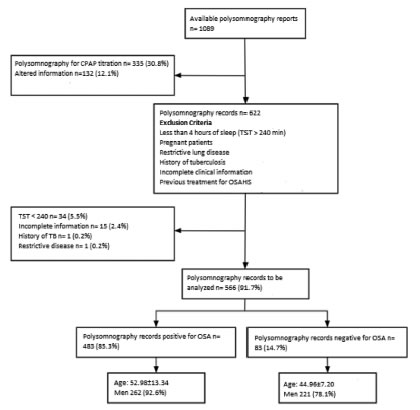
Mean age was 51.80 ± 13.73 years, and 50% were male. According to the polysomnography report, the general prevalence of OSA was 85.3% (483 of 566, 95% CI 82.4-88.35%), where the most frequent was mild OSA in 42.2% (AHI 8.4 ± 3.0), with similar behavior between moderate OSA (27.3% with AHI 21 ± 4.1) and severe OSA (30.5% with AHI 49.2 ± 18.7), with higher prevalence in men (92.6%, 95% CI 89.5-95.6%) than in women (78.1%, 95 % CI 73.3-89.5%, p < 0.001), showing a mean AHI of 26.05 ± 21.89 event/hour that increases when the patient is in supine position (AHI of 32.76 ± 25.82 event/hour).
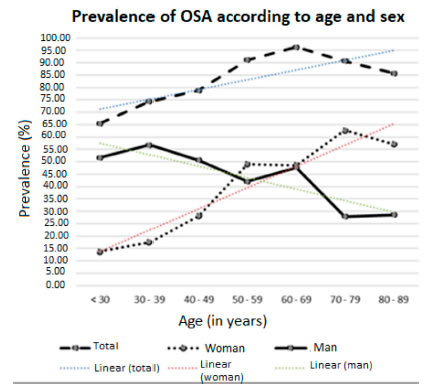
As shown in Figure 2, the general prevalence of OSA showed positive linear growth regarding age (p <0.001), a phenomenon that was also observed in women; men, on the other hand, showed the opposite behavior (negative linear growth, p < 0.001); only in the age group of 60 to 69 years the OSA distribution was the same for both men and women.
Tables 1 and 2 show the distribution of the study population and prevalence of OSA according to sociodemographic variables, comorbidities, and clinical and polysomnographic characteristics.
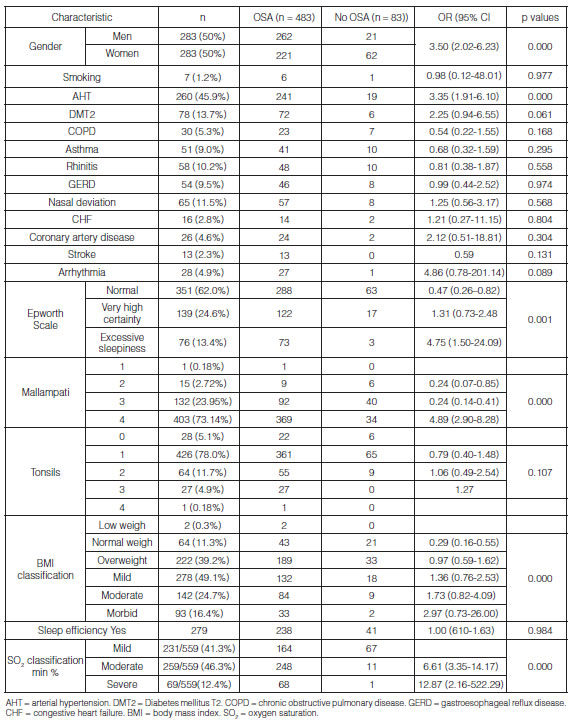
The analysis showed that the probability of having OSA is 3.5 times higher in men compared to women (95% CI 2.02-6.23, p < 0.000), 3.35 times higher in hypertensive patients (95% CI 1.91-6.10, p < 0.000), 4.89 times higher in patients with Mallampati grade IV (95% CI 2.90-8.28, p < 0.000) and 4.75 times higher in patients with excessive sleepiness (95% CI 1.50-24.09, p< 0.001). There were other important clinical variables such as diabetes mellitus (OR 2.25 95% CI 0.94-5.35, p < 0.061), arrhythmias (OR 4.86 95% CI 0.78-201.14, p < 0.089) and obesity (mild OR 1.36 95% CI 0.76-2.53, moderate OR 1.73 95% CI 0.82-4.09 and morbid OR 2.97 95% CI 0.73-26) with a higher probability of presenting the event, but these results are not statistically significant. One of the factors reducing the probability of the event is normal weight (OR 0.29 95% CI 0.16-0.55, P<0.000) and an Epsworth scale < 11 points, which means normality (OR 0.47 95% CI 0.26-0.82, p < 0.004), (Table 1).
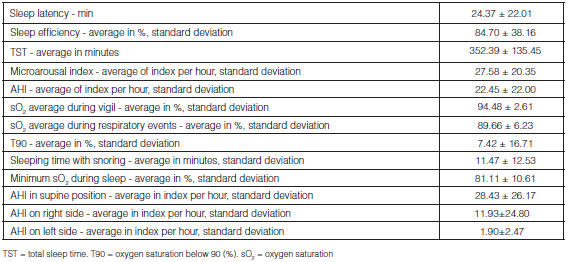
The average duration of sleep latency time was longer in the group without OSA (29.85 ± 23.47 min vs. 23.26±21.63 min, p < 0.005), not showing onset insomnia (> 30 minutes). Regarding the total sleep time (TST), the average duration of the OSA group was shorter than the group without OSA (346.60±41.50min vs 386.09 ± 339.03 min, p < 0.005); however, in terms of sleep efficiency, there weren’t statistically significant differences between the groups and the average was adequate, 84.70±38.16%. Patients with OSA showed higher desaturation in the different stages of sleep and during respiratory events, in comparison with patients without OSA (Table 1).
The multiple logistic regression analysis showed a model in which the predictor variables included gender, AHT, arrhythmias, Mallampati, BMI and Epsworth scale. It was identified that men (adjusted OR 4.46 95% CI 2.04-8.04, p < 0.000), hypertensive patients (adjusted OR 3.78 95% CI 2.48-8.04, p < 0.000), patients with Mallampati grade IV (adjusted OR 4.14 95% CI 2.41-7.10, p < 0.000) and patients with excessive sleepiness (adjusted OR 5.70 95% CI 1.66-19.53, p < 0.006) were significantly and independently associated with OSA. The lower probability of having OSA with normal weight was 52% (adjusted OR 0.48 95% CI 0.24-0.97, p < 0.043), setting this parameter as a protective factor. As summarized in Table 3, there wasn’t a significant association with other factors.
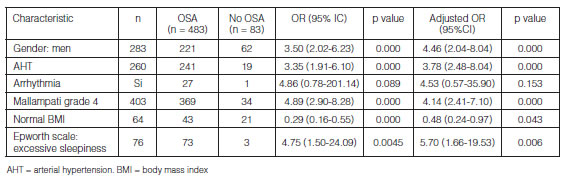
As shown in Figure 3, we evaluated the model’s degree of success of the event 3. compared to the real cases that were presented through a ROC curve with 86.35% of correct classification.
We conducted the Hosmer-Lemeshow test obtaining a non-significant p value of 0.4130, thus concluding that the model may be considered acceptable to explain the study data.
Discussion
This is the first research made in Cali about the frequency and factors associated with the presence of OSA in patients with high clinical suspicion. Our study showed 85.3% prevalence of OSA, in accordance with the information published by Morales et al in Bogotá indicating 75.7%22 prevalence in this kind of population. The possible cause of this phenomenon is selectivity of patients admitted to this specialized center, because they have high clinical suspicion before the test. Another relevant aspect is the identification of 45.9% of records with history of arterial hypertension, 92.7% of which have OSA, which may impact on the described prevalence.
Approximately 40% of hypertensive patients have OSA, reaching even 90% in patients with refractory hypertension6. In Cali, Colombia, González et al published 67.4% prevalence of OSA in the hypertensive population23, which is lower than the values observed in this study.
It has been reported that arterial hypertension increases the probability of having OSA, because during sleep healthy people reduce diastolic and systolic numbers of arterial tension between 10 to 15%, in comparison with the vigil status. This phenomenon is known as “dipping”. However, it has been observed that in patients with OSA this phenomenon is reduced during sleep, thus causing a higher cardiovascular risk24.
The Wisconsin Sleep Cohort, a study that began in 1988 with a random sample of 2,940 state employees in Wisconsin which provided essential data to estimate the prevalence of non-diagnosed breathing disturbances during sleep in adults and its importance for public health25 and The Sleep Heart Health, a multicentric study that began in 1995 including 6 cohorts already in process with a sample of 6,441 participants, designed to prove if breathing disturbances during sleep are associated with a higher risk of cardiovascular mortality and morbidity26 are the basis for understanding the natural history of sleep breathing disturbances, showing a positive linear relationship between the prevalence of hypertension and the severity of OSA, with a 2.7 times higher probability of showing hypertension if the disorder is mild and 4.5 times higher if it is moderate to severe.27 In our study, the adjusted probability of developing OSA is three times higher in hypertensive patients.
With regard to the age variable, aging is a factor of risk at its peak as a consequence of the greater longevity of the general population. At present, 1 every 4 polysomnographies done in Spain are performed in individuals of 65 years or older, due to a higher clinical suspicion in this population28. In Korea, a linear association was evidenced between age and the high risk of having OSA, which is higher in people of 60 years and older, where the prevalence is equaled between men and women29. Such behavior is evidenced in this study, with the difference of the negative linear growth seen in men.
Classically, obese and sleepy men represent the OSA phenotype30. As observed in this study, OSA is more prevalent in men than in women (92.6% vs. 78.1%, p <0.001), with a 4.5 times higher probability of developing OSA. This finding is corroborated in the multiple studies available up to now, with prevalence values between 13% and 33%1.
Obesity (mainly centripetal) has an important association with OSA, since it increases the pharyngeal collapsability by mechanical effects in the soft tissues of the pharynx and the restriction generated at the lung volume level, apart from affecting the neuromuscular control of the airways exercised by the central nervous system2. In this study we observe that the higher the degree of obesity, the greater the opportunity for the event; however, this result is not statistically significant, unlike what happens with normal weight, considered a protective factor.
The phenotype of excessive daytime sleepiness is most common in men and is related to an increase in the hypoxemic load (more apneas versus hypopneas) and is associated with neurocognitive, metabolic and cardiovascular impairment and a great impact on the quality of life31, using the Epworth scale to evaluate the frequency. It was positive in 1 every 8 evaluated records and was present in 96.1% of patients with OSA, as opposed to other studies where half the patients with sleep apnea had this condition32. Laranjeira et al established that the Epsworth sleepiness scale is not connected with the disease33. The same was observed in patients with moderate OSA, where the presence of excessive sleepiness was associated with a higher risk of hypertension, but interaction between the AHI and the Epsworth score wasn’t statistically significant34. In this case, the logistic regression model informs that having intense sleepiness provides 4.7 times higher probability of having OSA and a scale in the normality range provides a protective factor for the event.
The anthropometric test is a secondary tool used for making the clinical diagnosis of OSA during the physical examination. Few of those tools provide a direct correlation with the presence or severity of
the disease, except for the Mallampati classification35, given that with Mallampati grade III or IV the relative risk of having OSA is 1.95 times, and 2.45 times if we add nasal obstruction36. In this study, Mallampati grade IV provides a 3.14 times higher probability of having OSA.
The physiopathological phenomena present during OSA could explain the independent association with other comorbidities such as T2 diabetes, where intermittent hypoxemia and sleep fragmentation lead to a modification of glucose metabolism, reaching 17% reduction of insulin sensitivity and an increase in plasma glucose levels without changes in insulin secretion37. But we should take into account the confusion effects that may be generated by aging and obesity in the bidirectional association and the reverse causality between breathing disturbances during sleep and T2 diabetes38.
Strausz et al showed an association with OSA in patients with T2 regardless of obesity, indicating that the risk of diabetic kidney disease increases 1.75 times in patients with OSA, as well as mortality for all the causes. In our study, despite the fact that T2 diabetes increases 2.3 times the probability of having OSA, results weren’t statistically significant39.
It has been shown that patients with severe OSA had higher rates of heart arrythmia40, 3 times higher probability of atrial fibrillation, 2 times higher probability of non-sustained ventricular tachycardia and almost double the probability of complex ventricular ectopy (bigeminy, trigeminy, quadrigeminy)41. In our study we can observe a 4 times higher probability of having OSA when heart arrhythmias are detected, but results weren’t statistically significant.
The strength of the study is the degree of success and plausibility of the model that was found which explains the data obtained, possibly due to the number of records included, much higher than those reported in other studies of this type. On the other hand, given the limited information available on a national level, this type of study allows us to know more about the local difficulties, having as limiting factor the fact that the behavior of the event is only shown inside a specialized medical center; results can’t be replicated in other populations.
Despite the model’s degree of success, there is residual confusion not allowing us to provide a full explanation of this disease; due to the retrospective condition of the study it is impossible to make an analysis of other variables that could possibly intervene and are not available in the polysomnographic records or in the given clinical records, thus generating an information bias. So, further prospective, preferably multicentric research is needed that allows for other variables such as risk assessment questionnaires, additional anthropometric tests, quality of life, anxiety and depression and the different characteristics of possible clinical phenotypes of sleep obstructive apnea, amongst other things, with the purpose of generating a hypothesis about the event.
OSA is a complex and heterogeneous disorder, since it depends on multiple elements such as risk factors, clinical physiopathological characteristics and comorbid conditions and not simply on the polysomnographic results, because the AHI alone is not enough to make a diagnosis, not producing all the necessary tools for the approach and treatment of this entity42.
Conclusion
OSA is common in this population possibly because it is influenced by the prevalence of arterial hypertension, which has proven to increase the probability of having this disease. It can be explained to a large extent by clinical variables such as gender, weight, blood pressure, Mallampati classification and excessive sleepiness, all of them independently associated. Knowing these determinant factors will allow clinicians to suspect the presence of OSA.
This research provides knowledge of determinant factors within a local population with OSA. With this information, it would be possible to generate a proposal to develop diagnostic algorithms that could approximate to the results obtained with the polysomnography, the gold standard for the diagnosis.
Acknowledgement
We wish to thank AIREC Specialized Medical Group of the city of Cali, Colombia and the statistical specialist Andrés Mauricio Castro for their collaboration in this document. We also thank all the teachers of the Epidemiology master’s degree and postgraduate degree in Internal Medicine leaded by the coordinator María Eugenia Casanova V., of the Universidad Libre (Seccional Cali) who not only provides and encourages knowledge but also “humanity, humor and humility”, as taught by William Osler, father of internal medicine.
Funding sources
Funded by the Universidad Libre Seccional Cali
Conflict of interest
The authors declare that there isn’t any kind of conflict of interest.
Funding
1. Hamilton GS, Matheson MC, Dharmage SC, et al. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med Rev. 2016; 34: 70-81. doi:10.1016/j.smrv.2016.07.002
2. LAM JCM, MAK JCW, IP MSM. Obesity, obstructive sleep apnoea and metabolic syndrome. Respirology. 2012; 17(2): 223-36. doi:10.1111/j.1440-1843.2011.02081.x
3. Hla KM, Young T, Hagen EW, et al. Coronary Heart Disease Incidence in Sleep Disordered Breathing: The Wisconsin Sleep Cohort Study. Sleep. 2015; 38(5): 677-84. doi:10.5665/sleep.4654
4. Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive Sleep Apnea–Hypopnea and Incident Stroke. Am J Respir Crit Care Med. 2010; 182(2): 269-77. doi:10.1164/rccm.200911-1746OC
5. Digby GC, Baranchuk A. Sleep apnea and atrial fibrillation; 2012 update. Curr Cardiol Rev. 2012; 8(4): 265-72. doi:10.2174/157340312803760811
6. Harding SM. Resistant Hypertension and Untreated Severe Sleep Apnea: Slowly Gaining Insight. J Clin Sleep Med. 2014; 10(8):845. doi:10.5664/JCSM.3948
7. Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in Patients with Chronic Obstructive Pulmonary Disease and Obstructive Sleep Apnea. Am J Respir Crit Care Med. 2010; 182(3): 325-31. doi:10.1164/rccm.200912-1869OC
8. Viswanathan V, Ramalingam IP, Ramakrishnan N. High Prevalence of Obstructive Sleep Apnea among People with Type 2 Diabetes Mellitus in a Tertiary Care Center. J Assoc Physicians India. 2017; 65(11): 38-42. http://www.ncbi.nlm.nih.gov/pubmed/29322708. Accessed April 24, 2019.
9. Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and Regression of Sleep-Disordered Breathing With Changes in Weight. Arch Intern Med. 2005; 165(20): 2408. doi:10.1001/archinte.165.20.2408
10. Benedetto M De, Garbarino S, Sanna A. Le Sindrome dell’Apnea Ostruttiva nel Sonno (OSAS): i costi sanitari e sociali. Med Lav. 2017; 108(4): 310-3. doi:10.23749/MDL.V108I4.6411
11. Testelmans D, Vrijsen B, Jennum P, et al. Definition, discrimination, diagnosis and treatment of central breathing disturbances during sleep. Eur Respir J. 2016; 49(1): 1600959. doi:10.1183/13993003.00959-2016
12. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The Occurrence of Sleep-Disordered Breathing among Middle- Aged Adults. N Engl J Med. 1993; 328(17): 1230-5. doi:10.1056/NEJM199304293281704
13. Sateia MJ. International classification of sleep disorders-third edition highlights and modifications. Chest. 2014; 146(5): 1387-94. doi:10.1378/chest.14-0970
14. Laratta CR, Ayas NT, Povitz M, Pendharkar SR. Diagnosis and treatment of obstructive sleep apnea in adults. CMAJ. 2017; 189(48): E1481-8. doi:10.1503/cmaj.170296
15. Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of Sleep Apnea Syndrome in U.S. Communities. Sleep Breath. 2002; 6(2): 49-54. doi:10.1055/s-2002-32318
16. Gonzaga C, Bertolami A, Bertolami M, Amodeo C, Calhoun D. Obstructive sleep apnea, hypertension and cardiovascular diseases. J Hum Hypertens. 2015; 29(12): 705-12. doi:10.1038/jhh.2015.15
17. Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000; 320(7233): 479-82. doi:10.1136/BMJ.320.7233.479
18. Chung F, Memtsoudis SG, Ramachandran SK, et al. Society of Anesthesia and Sleep Medicine Guidelines on Preoperative Screening and Assessment of Adult Patients With Obstructive Sleep Apnea. Anesth Analg. 2016; 123(2): 452-73. doi:10.1213/ ANE.0000000000001416
19. Kaw R, Chung F, Pasupuleti V, Mehta J, Gay PC, Hernandez AV. Meta-analysis of the association between obstructive sleep apnoea and postoperative outcome. Br J Anaesth. 2012; 109(6): 897-906. doi:10.1093/bja/aes308
20. Hirsch Allen AJ, Park JE, Daniele PR, Fleetham J, Ryan CF, Ayas NT. Obstructive sleep apnoea and frequency of occupational injury. Thorax. 2016; 71(7): 664-6. doi:10.1136/thoraxjnl-2015-207994
21. Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009; 5(6): 573-81. http://www.ncbi.nlm.nih.gov/pubmed/20465027. Accessed March 13, 2019.
22. Morales Á, Hidalgo Martínez P, Amado Garzón S, Medina López L. Prevalencia de síndrome metabólico y obesidad en pacientes con síndrome de apnea hipopnea del sueño (SAHOS) en el Hospital Universitario San Ignacio. Rev Colomb Neumol. 2018; 24(1):1 8. doi:10.30789/rcneumologia.v24.n1.2012.200
23. Hernández G, Maria L, Castrillón C, Jaime J, García H. Available at: http://www.redalyc.org/articulo.oa?id=273820368002.2008.
24. Golbin JM, Somers VK, Caples SM. Obstructive sleep apnea, cardiovascular disease, and pulmonary hypertension. Proc Am Thorac Soc. 2008; 5(2): 200-6. doi:10.1513/pats.200708-143MG
25. Young T. Rationale, design and findings from the Wisconsin Sleep Cohort Study: Toward understanding the total societal burden of sleep disordered breathing. Sleep Med Clin. 2009; 4(1): 37-46. doi:10.1016/j.jsmc.2008.11.003
26. Wahl PW. Quan SF, Howard BV, et al, Rapoport DM, Redline S, Robbins J SJ. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997; 20(12): 1077-85.
27. Peppard PE, Young T, Palta M, Skatrud J. Prospective Study of the Association between Sleep-Disordered Breathing and Hypertension. N Engl J Med. 2000; 342(19): 1378-84. doi:10.1056/NEJM200005113421901
28. Martinez-Garcia MA, Valero-Sánchez I, Reyes-Nuñez N, et al. Continuous positive airway pressure adherence declines with age in elderly obstructive sleep apnoea patients. ERJ open Res. 2019;5(1). doi:10.1183/23120541.00178-2018
29. Sunwoo J-S, Hwangbo Y, Kim W-J, Chu MK, Yun C-H, Yang KI. Prevalence, sleep characteristics, and comorbidities in a population at high risk for obstructive sleep apnea: A nationwide questionnaire study in South Korea. PLoS One. 2018; 13(2): e0193549. doi:10.1371/journal.pone.0193549
30. Verbraecken J, Farre R, Martinez-Garcia M-A, et al. Challenges and perspectives in obstructive sleep apnoea. Eur Respir J. 2018; 52(3): 1702616. doi:10.1183/13993003.02616-2017
31. Chervin RD, Aldrich MS. Characteristics of apneas and hypopneas during sleep and relation to excessive daytime sleepiness. Sleep. 1998; 21(8): 799-806. doi:10.1093/sleep/21.8.799
32. Budhiraja R, Kushida CA, Nichols DA, et al. Predictors of sleepiness in obstructive sleep apnoea at baseline and after 6 months of continuous positive airway pressure therapy. Eur Respir J. 2017; 50(5). doi:10.1183/13993003.00348-2017
33. Laranjeira C, Barbosa E, Rabahi M. Is subjective sleep evaluation a good predictor for obstructive sleep apnea? Clinics. 2018; 73(25): 1-7. doi:10.6061/clinics/2018/e355
34. O’Connor GT, Caffo B, Newman AB, et al. Prospective Study of Sleep-disordered Breathing and Hypertension: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2009; 179(12): 1159. doi:10.1164/RCCM.200712-1809OC
35. Friedman M, Hamilton C, Samuelson CG, Lundgren ME, Pott T. Diagnostic Value of the Friedman Tongue Position and Mallampati Classification for Obstructive Sleep Apnea. Otolaryngol Neck Surg. 2013; 148(4): 540-7. doi:10.1177/0194599812473413
36. Liistro G, Rombaux P, Belge C, Dury M, Aubert G, Rodenstein DO. High Mallampati score and nasal obstruction are associated risk factors for obstructive sleep apnoea. Eur Respir J. 2003; 21(2): 248-52. doi:10.1183/09031936.03.00292403
37. Newhouse LP, Joyner MJ, Curry TB, et al. Three hours of intermittent hypoxia increases circulating glucose levels in healthy adults. Physiol Rep. 2017; 5(1). doi:10.14814/phy2.13106
38. Reutrakul S, Mokhlesi B. Obstructive Sleep Apnea and Diabetes: A State of the Art Review. Chest. 2017; 152(5): 1070. doi:10.1016/J.CHEST.2017.05.009
39. Strausz S, Havulinna AS, Tuomi T, et al. Obstructive sleep apnoea and the risk for coronary heart disease and type 2 diabetes: a longitudinal population-based study in Finland. BMJ Open. 2018;8(10):e022752. doi:10.1136/BMJOPEN-2018-022752
40. Vijayan VK. Morbidities associated with obstructive sleep apnea. Expert Rev Respir Med. 2012; 6(5): 557-66. doi:10.1586/ ers.12.44
41. Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006; 173(8): 910-6. doi:10.1164/rccm.200509-1442OC
42. Zinchuk A V., Gentry MJ, Concato J, Yaggi HK. Phenotypes in obstructive sleep apnea: A definition, examples and evolution of approaches. Sleep Med Rev. 2017; 35: 113-23. doi:10.1016/j.smrv.2016.10.002