Autor : Vicente Antonela1, Amoza RocĂo L.1, GarcĂa Reid Cecilia1, Tocalini Pablo1, Prieto Luciana1, Savio Paula1, Simioni MarĂa Belen1, Ferrario Antonela1, Cura Adriano J.1, Tozzi Walter A.1, Villarruel MatĂas1, Verde Gabriel A2, Garegnani Luis I.1 Virgilio Sacha A.1
1 Bachelor’s Degree in Kinesiology and Physiatry, Hospital General de Agudos Parmenio T. Piñero; Av. Varela 1301. Zip Code 1406. Autonomous City of Buenos Aires. 2 Intensive care Physician and Head of the Intensive Care Unit of the Hospital General de Agudos Parmenio T. Piñero; Av. Varela 1301. Zip Code 1406. Autonomous City of Buenos Aires.
Correspondencia : Lic. Antonela Vicente. E-mail: antovicen@hotmail.com . ORCID ID: https://orcid.org/0000-0003-3152-845X
Abstract
Introduction: Intensive care unit-acquired weakness (ICUAW) affects the muscles of the limbs and diaphragm; and is associated with negative outcome variables. The purpose of this study was to describe the epidemiological characteristics and incidence of ICUAW in adults requiring invasive mechanical ventilation (IMV) for more than 72 hours in a General Hospital for Acute Diseases in the City of Buenos Aires.
Method: Retrospective cohort study. We included adult patients requiring IMV for more than 72 hours in the Intensive Care Unit (ICU). The main recorded variables of interest were: incidence of ICUAW, days of IMV, length of stay and mortality both in the ICU and in the hospital.
Results: 262 patients were included in the study; 87 of them (33.21%) developed weakness. No statistically significant differences were established between patients with and without ICUAW regarding the variables of age, reason for admission to IMV, medical history and mortality both in the ICU and the hospital.
In this study, the variables established as independent risk factors were: female gender (OR: 1,98; 95% CI: 1.02-3.81), delirium (OR 8.4; 95% CI: 4.38-16.11) and days of IMV (OR: 1.05; 95% CI: 1.02-1.08).
Conclusions: This study allowed us to know the incidence and epidemiological characteristics of patients with ICUAW in an ICU of the public health system of Argentina. It was observed that female gender, days of IMV and delirium at the ICU were independent risk factors for ICUAW.
Key words: Polyneuropathies; Muscular Strength; Artificial Respiration; Intensive Care Unit and Epidemiology.
Introduction
Invasive mechanical ventilation (IMV) is a therapeutic resource of vital support that has allowed the favourable modification of the prognosis of critically ill patients, but its prolonged use usually causes complications1. One of them is the intensive care unit-acquired weakness (ICUAW), characterized by generalized limb muscle weakness, with the patient critically ill2. There is also respiratory compromise, due to the alteration of the diaphragm and phrenic nerves3.
Various studies have shown the negative impact generated by the ICUAW, producing weaning difficulties3, increasing IMV days and the length of stay in the intensive care unit (ICU) and in the hospital5,6 and even increased mortality6-8.
ICUAW detection has been increasing in the last years, associated with the increased survival of critically ill patients4. Stevens et al made a systematic review (SR) where they informed a 46% incidence9. On the other hand, the Argentinian study performed at the Hospital Posadas reported a 40.5% incidence in patients requiring IMV for more than 24 hours10.
There are different diagnostic methods for assessing the neuromuscular function, the most recommended being the clinical diagnosis. The muscle biopsy is considered the gold standard, but it has the disadvantage of being invasive and is recommended in those cases where it is impossible to carry out an electrophysiological study and where another cause of weakness is suspected11,12. Such study is not largely used because it requires an experienced technician and the correct equipment at the ICU10. That is why from the clinical point of view a widely accepted simple tool for detecting the ICUAW is the Medical Research Council (MRC) Sum-Score, used to assess muscle strength. It has been used in critically ill patients suffering from that condition9,11. The purpose of this tool is to individually and bilaterally quantify weakness of the muscle groups of the upper and lower limbs, with good inter-observer reliability (intraclass correlation coefficient: 0.83)12.
Multiple studies have researched the risk factors associated with this condition, some of them are: sepsis9, shock10-13 and multiple organ failure9, 10, 12; whereas other studies found an association with the variables age, duration of IMV, ICU9, 11 length of stay, delirium, hyperglycemia, use of corticosteroids and neuromuscular blocking agents12-15.
Information regarding the evolution and characteristics of these patients in our country is scarce. A retrospective study was recently published of the characteristics of patients who developed ICUAW at our ICU during a period of three years. The results obtained showed that age, delirium, IMV days and hospital stay were statistically significant in subjects with weakness, not taking into account confounding variables14. For that reason and with the purpose of improving the methodological quality of the study14, we decided to conduct a multivariate logistic regression analysis.
So, the purpose of this study is to describe the epidemiological characteristics and incidence of ICUAW in adult patients requiring IMV for more than 72 hours in the ICU of a General Hospital for Acute Diseases in Buenos Aires. Secondarily, to conduct a comparative analysis of the main variables under evaluation between subjects who developed ICUAW and subjects who did not, subsequently identifying independent risk factors for the development of ICUAW.
Materials and Methods
We conducted a retrospective cohort study from July 1, 2012 until December 31, 2016 inclusive. The study consecutively included adult patients requiring IMV for more than 72 hours at a medical-surgical ICU15 with 8 available beds and specialized staff: critical care physicians, nurses and physiotherapists available 24 hours a day.
This study was approved by the Bioethics Committee.
Patients with the following characteristics were excluded:
– Lesions of the brainstem, cerebellar and/or uni- or bihemispheric cerebral lesions16 (during the study period or before) documented in the medical record, whether it is a stroke, a traumatic brain injury (TBI), etc.
– Lesions of the spinal cord and/or pre-existing neuromuscular disorder documented in the medical record.
– Peripheral nerve diseases, amputation and/or fractures compromising more than two limbs.
– Diseases that affect the comprehension skills16.
– Patients who weren’t tested for ICUAW (unrecorded data).
The following variables were defined and collected:
– Birth data and history of every patient included in the study.
– Incidence: relationship between patients with ICUAW and the total number of patients included in the study.
– Intensive care unit-acquired weakness: patients who met the criteria for the evaluation underwent daily screening taking into account their awakening and comprehension basing on their response to five commands: “open and close your eyes”, “look at me”, “open your mouth and stick out your tongue”, “nod” and “lift your eyebrows when I count to five” (16). Once the patient responded to the five commands, he/she was considered awake and the MRC Sum-Score test was performed. We continued with the bilateral assessment of three muscle groups of the upper limb: Shoulder abductors, elbow flexors and wrist extensors; and three groups of the lower limb: hip flexors, knee extensors and ankle dorsal flexors17. Once the individual scores were obtained, the twelve muscle groups were added.
The MRC Sum-Score scale gives a 0 score to muscle groups with paralysis and 5 if there is normal muscle strength, with a maximum score of 6016,17,20. The cut-off point to consider ICUAW is <48 points16,17.
– Reason for starting IMV: 18, 19
o Exacerbated chronic respiratory failure (CRF): chronic obstructive pulmonary disease (COPD), asthma or other.
o Acute respiratory failure (ARF): adult respiratory distress syndrome (ARDS), postoperative (PO), heart failure, aspiration, pneumonia, sepsis, polytrauma, cardiac arrest or other.
o Coma: metabolic, due to intoxication or other.
– Delirium: evaluated on a daily basis by the physiotherapist whenever the patient presented a value between -3 and +4 according to the Richmond Agitation and Sedation Scale (RASS). The Confusion Assessment Method Intensive Care Unit (CAM- ICU) test was used, according to the Chilean validation21.
– Weaning type and method: the moment the treating team considered the patient ready to tolerate spontaneous ventilation18, 19 was recorded as the beginning of the weaning process. Patients were classified in three groups basing on the duration and difficulty of weaning (simple, difficult and prolonged), according to the classification by Boles et al22.
– Reintubation (reIOT): defined as the need to restore the artificial airway within 48 hours after extubation22.
– Tracheostomy (TQT): it was recorded the day the procedure was carried out, and the percentage of patients requiring it in each group was calculated.
The following temporary and mortality variables were recorded15:
– Total number of IMV days: calculated from the moment it began to be used at the ICU (day 0) until successful weaning and extubation, referral to another institution or the patient´s death.
– ICU length of stay: calculated from the day the patient was admitted (day 0) until medical discharge, death or referral to another unit and/or institution.
– Hospital length of stay: calculated from hospital admission (day 0) until discharge, referral to another institution or the patient´s death.
– ICU and hospital mortality: it was recorded according to the vital status of the patient upon hospital discharge.
Statistical Analysis
The continuous variables were reported according to the distribution as mean and standard deviation or median and interquartile range 25-75. For the comparison of the mean values we used the Student Test or Mann-Whitney-Wilcoxon U Test, as appropriate according to the distribution of variables. The categorical variables were expressed in percentage per groups and the values were compared through the X2 test. The multivariate analysis was carried out with logistic regression, with an adjustment model, where variables related to the variable of interest were kept within the model.
The variables that showed p < 0.20 in the univariate analysis and those related to ICUAW results were included one by one in the multivariate analysis. Variables that maintained a significant association or were considered possible risk factors related to the variable of interest were used for the multivariate analysis. We evaluated the presence of possible effect modification variables. We analyzed two categories of the age variable, according to the terms established in the literature20, less than or equal to and more than 64 years. Colinearity was observed between the variables “days of IMV” and “ICU length of stay”. For the final model, the “days of IMV” variable was selected.
Model calibration was evaluated with the Hosmer-Lemeshow test. Global discrimination was considered from the area under the ROC (receiver operating characteristic) curve and from a classification table with a cut-off point of 40% risk of showing ICUAW. Results observed in the univariate and multivariate models were showed in a table. Odds Ratio (OR) and 95% Confidence Interval (CI) values were expressed.
All the statistical tests were two-tailed with a statistical significance of p < 0.05.
For the whole statistical analysis we used STATA/MP Version 14.0.
Results
281 out of all the patients (n=604) admitted to the ICU in the study period were ready to be assessed by means of the MRC Sum-Score. Since there was unrecorded data of 19 subjects, finally 262 patients were analyzed (Figure 1). The incidence of ICUAW was 33.21% (87/262).
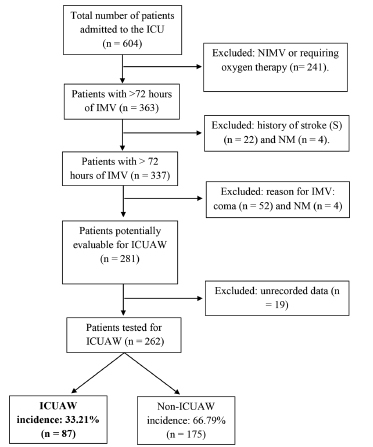
Table 1 details the demographic characteristics of patients admitted to the study.
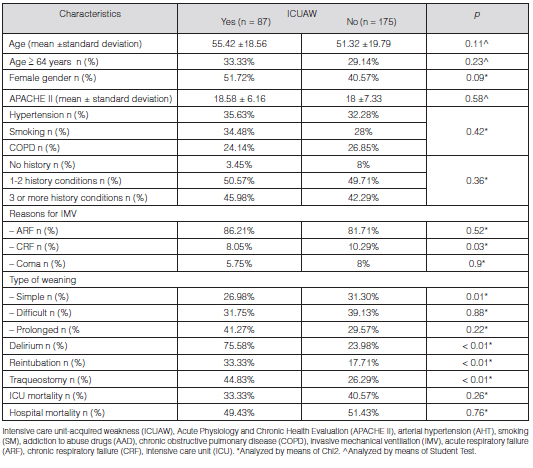
We conducted a univariate analysis between subjects with and without ICUAW who received IMV, and found statistically significant differences for the variables female gender, reasons for IMV: CRF subgroup, simple weaning, delirium, reIOT rate and TQT (Table 1).
Temporary variables were expressed through the Box Plot (Figure 2), where statistically significant differences were found (p < 0.001) regarding the variables IMV days and ICU and hospital length of stay.

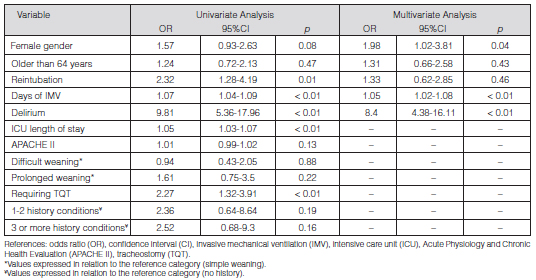
In the logistic regression multivariate analysis there were variables independently associated with ICUAW: female gender (OR 1.98; 95% CI 1.02-3.81; p = 0.04), delirium (OR 8.4; 95% CI 4.38-16.11; p < 0.001) and IMV days (OR 1.05; 95% CI 1.02-1.08; p < 0.001) (Table 2). The final model showed 0.83 calibration measured through the Hosmer-Lemeshow test. The discriminative capacity was evaluated by means of the area under the curve: 0.82 (95% CI; 0.76-0.88), with a classification capacity of 78.21%, basing on the 40% ICUAW cut-off point. 73.26% sensitivity and 80.7% specificity were found. Different cut-off values were used, with the same classification capacity.
The Kaplan-Meier curve (Figure 3) represented the survival of subjects with and without ICUAW. A 36-day median survival was found in the group with ICUAW and 16 days for subjects without ICUAW.

Discussion
The incidence of ICUAW was 33.21% lower than the values provided by Stevens et al9, who reported 46%. The studies analyzed in such SR used electrophysiology to confirm the diagnosis9, whereas this study used clinical assessment. This difference could be reflected in the variation of incidence, since the clinical method requires the patient’s cooperation and understanding, thus limiting the performance of the evaluation. The electrophysiological study, on the other hand, may detect variations at an early stage (24-48 hours) in sedated or comatose patients23. Also, the SR of Stevens et al9 included studies at heterogeneous ICUs, different methodological designs and different inclusion criteria.
Even though ICUAW subjects were older, age was not an independent risk factor, just like in the study of De Jonghe et al20. The mean age for the group of ICUAW patients in this study was similar to that reported by Diaz Ballve et al10 (55.42 ± 18.56 and 55.9 ± 17.6 respectively), but in that study age was an independent risk factor for the development of this weakness. The reason for this difference could be the fact that the Argentinian study analyzed variables not taken into account in this study, such as sedative and vasopressor days, renal failure days, hyperglucemia, corticosteroid therapy, among other things. Also in the Diaz Ballve et al10 study, the age variable was expressed continuously and without the cut-off point suggested by De Jonghe et al20 used in this study.
Regarding the female gender, it was found as an independent risk factor for developing ICUAW, the same as the results provided by De Jonghe et al16, who believe there isn’t a clear explanation for this finding. Those authors proposed as possible hypotheses the pharmacokinetic differences between male and female patients, and suggested that the latter would show lower muscle strength values16.
The intercurrent condition “delirium” is an independent predictor of mortality, more IMV days and longer hospital stay24. In this study it was found as an independent risk factor for developing ICUAW with an OR of 8.4 (95% CI; 4.38-16.11, p = 0.001), just like in the study of Diaz Ballve et al10, who reported an OR of 3.92 (95% CI; 1.57-9.74, p = 0.003). There is insufficient information about the relationship between delirium and ICUAW25, but patients who developed delirium and/or ICUAW were associated with more IMV days, prolonged ICU and hospital stay, more sedation, and increased mortality at the ICU, at the hospital and one year after being discharged10, 25-27.
No association could be established between ICUAW and the type of delirium; such information was not detailed in the medical records.
As for the TQT rate, the results obtained in this study coincide with the findings of the prospective cohort study of Garnacho Montero et al6, where more than half of the patients with ICUAW required TQT, unlike those who did not show weakness, where only 13% required TQT (p< 0.05). On the other hand, we couldn’t establish an association with the timing of the event due to the retrospective nature of the study.
We found a significant association between days of IMV and ICUAW, the same as twelve other studies included in the SR of Stevens et al9. In three of those studies6, 16, 27, also statistical significance was found in the multivariate analysis, just like the findings of Diaz Ballve et al10 and this study.
Unlike this study, De Jonghe et al.27 considered prolonged weaning in ICUAW patients to be an independent risk factor. However, in this study subjects without ICUAW showed a higher percentage of simple weaning with statistically significant differences, which is why they had fewer days with IMV and shorter length of ICU and hospital stay.
It is important to mention that mortality in this study was higher than the one reported by the international literature19 and the aforementioned according to the APACHE II values. This finding could be related to the fact that such score evaluates severity on ICU admission, but maybe it does not take into account the difficulty of the weaning process or the evolution of the patient during his/her stay in said unit. Also the greater amount of IMV days of both groups in comparison with the study of Esteban et al19 could be related to a higher risk of developing associated intercurrent conditions22 and consequently, higher mortality.
Hermans et al28 didn’t find an association between ICUAW and the variable mortality at the ICU and at the hospital, the same as the results reported in this study. However, Leijten et al found that 48% of subjects who developed ICUAW showed higher mortality at the ICU in comparison with the control group (19%; p = 0.03)29. The authors say that this finding could be associated with complications generated inside the ICU such as multiple organ failure, and not by the premorbid status of the patient on admission to the unit. They believe that the prolonged critical disease plus the complications increase the risk of developing weakness, considering it within the physiopathology of multiple organ failure.
The univariate analysis showed that the variable reIOT had statistically significant differences in subjects with ICUAW (p= 0.005). These results are similar to the findings of Thille et al5, where patients showing intercurrent weakness had a higher probability of suffering extubation failure (p = 0.019).
De Jonghe et al.24 assessed survival through the Kaplan-Meier curve for subjects with and without ICUAW. It is important to say that the author included patients requiring IMV ≥ 7 days and considered the first day to be the day they woke up satisfactorily, unlike this study which admitted patients requiring IMV > 72 hours and considered the zero day the moment when the IMV was placed.
Limitations
The international literature reports various risk factors for developing ICUAW, some of which were not included in this study, given that they weren’t recorded in the data collection sheet, for example: hyperglycemia7,10,17, immobilization17, use of glucocorticosteroids7, 16 and neuromuscular blocking agents7, 19.
The main study variable was analyzed in a dichotomous way, making it impossible to obtain the mean value of the MRC Sum-Score.
Conclusion
This study allowed us to know the epidemiological characteristics and incidence of ICUAW in adult patients requiring IMV for more than 72 hours at the ICU of the Hospital General de Agudos Parmenio T. Piñero.
The multivariate analysis showed that the female gender, days of IMV and delirium behaved as independent risk factors for developing ICUAW. Prospective studies are required to analyze this population of patients admitted to the ICUs of Argentina.
Conflict of Interest and Funding
- None of the previously mentioned authors received grants, equipment, drugs and/or any other support contributing to the research or writing of the original text. We do not have any conflict of interest.
Acknowledgement
– Intensive Care Unit and Physiatry Service of the Hospital General de Agudos Parmenio T Piñero.
– Statistical and methodological advisor: Sacha A Virgilio.
1. Chiappero G y Villarejo F. (2010). Ventilación Mecánica, Libro del Comité de Neumonología Crítica de la Sociedad Argentina de Terapia Intensiva (SATI). Buenos Aires, Argentina: Editorial Panamericana.
2. Jung B, Moury PH, Mahul M, et al. Diaphragmatic dysfunction in patients with ICU-acquired weakness and its impact on extubation failure. Intensive Care Med. 2016; 42(5): 853-61.
3. Hermans G, De Jonghe B, Bruyninckx F, Van den Berghe G. Interventionsfor preventing critical illness polyneuropathy and critical illness myopathy (Review). Cochrane Database Syst Rev 2014, Issue 1. Art. No: CD006832.
4. Powers SK, Kavazis AN, Levine S. Prolonged mechanical ventilation alters diaphragmatic structure and function. Crit Care Med. 2009; 37 (10 Suppl): S347-53.
5. Thille A, Boissier F, Ghezala B, Razazi K, Mekontso-Dessap A, Brun-Buisson C. Risk Factors for and Prediction by Caregivers of Extubation Failure in ICU Patients: A Prospective Study. Crit Care Med 2015; 43: 613–20.
6. Garnacho-Montero J, Amaya-Villar R, García Garmendía JL, Madrazo-Osuna J, Ortiz-Leyba C. Effect of critical illness polyneuropathy on the withdrawal from mechanical ventilation and the length of stay in septic patients. Crit Care Med 2005; 33: 349-54.
7. De Jonghe B, Lacherade JC, Sharshar T, Outin H. Intensive care unit-acquired weakness: Risk factors and prevention. Crit Care Med 2009; 37: 309-15.
8. van Wagenberg L, Witteveen E, Wieske L, Horn J. Causes of Mortality in ICU-Acquired Weakness. J Intensive Care Med. 2017; 35(3): 293-6.
9. Stevens RD, Dowdy DW, Michaels RK, Mendez-Tellez PA, Pronovost PJ, Needham DM. Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med 2007; 33: 1876-91.
10. Ballve L, Dargains N, García Urrutia J, Bratos A, Percaz M, Bueno Ardariz C. Debilidad adquirida en la unidad de cuidados intensivos. Incidencia, factores de riesgo y su asociación con la debilidad inspiratoria. Estudio de cohorte observacional. Rev Bras Ter Intensiva. 2017; 29(4): 466-475.
11. Ibarra-Estrada MA, Briseño-Ramírez J, Chiquete E, Ruiz-Sandoval JL. Debilidad adquirida en la Unidad de Cuidados Intensivos: Polineuropatía y miopatía del paciente en estado crítico. Revista Mexicana de Neurociencia. Julio-Agosto, 2010; 11(4): 289-95.
12. Vanpee G, Hermans G, Segers J, Gosselin R. Assessment of Limb Muscle Strength in Critically Patients: A Systematic Review. Crit Care Med 2014; 42: 701-11.
13. Horn J, Hermans G. Intensive care unit-acquired weakness. In: Handbook of Clinical Neurology. 2017; 141: 531-43.
14. Cura A, Tozzi W, Ali M, et al. Characteristics of Patients with Intensive Care Unit Acquired Weakness of the Acute General Hospital “Parmenio Pinero”. Retrospective Study. Int J Clin Case. 2018; 2: 1-3.
15. Giménez ML, Verde GA, Salvati IG, et al. Características de los pacientes desvinculados de la ventilación mecánica invasiva. Rev Am Med Resp 2016; 2: 105-12.
16. De Jonghe B, Sharshar T, Lefaucheur JP, et al. Paresis Acquired in the Intensive Care Unit. A Prospective Multicenter Study. JAMA 2002; 288: 2859-67.
17. Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care. 2015; 19: 274.
18. Esteban A, Anzueto A, Frutos F, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002; 287(3): 345-55.
19. Esteban A, Frutos-Vivar F, Muriel A, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med. 2013; 188(2): 220-30.
20. De Jonghe B, Bastuji-Garin S, Durand MC, et al. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med 2007; 35: 2007-15.
21. Tobar E, Romero C, Galleguillos T, et al. [Confusion Assessment Method for diagnosing delirium in ICU patients (CAM-ICU): cultural adaptation and validation of the Spanish version]. Med Intensiva. 2010; 34(1): 4-13.
22. Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur. Respir. Jour. 2007; 29(5): 1033-56.
23. Fan E. Critical illness neuromyopathy and the role of physical therapy and rehabilitation in critically ill patients. Respir Care. 2012; 57(6): 933-44.
24. Aparecida Leite M, Osaku EF, Rejane Lima de Macedo Costa C, et al. Delirium during Weaning from Mechanical Ventilation. Crit Care Res and Pract. Volume 2014, Article ID 546349, 7 pages.
25. Latronico N, Herridge M, Hopkins RO, et al. The ICM research agenda on intensive care unit-acquired weakness. Intensive Care Med. 2017;43(9): 1270-81.
26. Garnacho-Montero J, Madrazo-Osuna J, García-Garmendia JL, et al. Critical illness polyneuropathy: risk factors and clinical consequences: a cohort study in septic patients. Intensive Care Med 2001; 27: 1288-96.
27. De Jonghe B, Bastuji-Garin S, Sharshar T, et al. Does ICU-acquired paresis lengthen weaning from mechanical ventilation?. Intensive Care Med. 2004; 30: 1117–21.
28. Hermans G, Van Mechelen H, Bruyninckx F, et al. Predictive value for weakness and 1-year mortality of screening electrophysiology tests in the ICU. Intensive Care Med. 2015; 41(12): 2138-48.
29. Leijten FS, Harinck de Weerd JE, Poortvliet DC et al. The role of polyneuropathy in motor convalescence after prolonged mechanical ventilation. JAMA 1995; 274: 1221-5.