Autor : Lugaro MartĂn C.1, 2, RĂos Fernando2, 3, Lauria VerĂłnica1, Jimenez Silvia1, Benito Mori Lilia1, Schoon Pablo1
1 Hospital General de Agudos “Prof. Dr. Luis Güemes”, Haedo, Buenos Aires 2 Sanatorio Las Lomas, San Isidro, Buenos Aires. 3 Hospital Nacional “Profesor Alejandro Posadas”, Haedo, Buenos Aires
Correspondencia : MartĂn Lugaro martinlugaro@gmail.com
Abstract
Introduction: The Fiberoptic Endoscopic Evaluation of Swallowing (FEES) is a technique
that allows the study of the physiology of swallowing. This technique can be applied at the
patient’s bedside, making it a very attractive choice for the critical care unit (CCU), since it
is not necessary to transfer the patient to another place in order to carry out the evaluation.
Objective: Feasibility to carry out the FEES at the patient’s bedside at the CCU and
assess the incidence of swallowing disorders in extubated patients.
Materials and Methods: Comparative, prospective, analytical cohort study conducted
24 hours after extubation for a period of 6 months, including consecutively all the patients
who received mechanical ventilation for a period ≥ 48 hours. The enrollment began in
March, 2015.
Results: 31 patients were included in the protocol. The incidence of swallowing disorders in extubated patients who required mechanical ventilation (MV) was 58%, 95%
CI [confidence interval] (0.407-0.735) with 18 patients presenting disorders out of 31
evaluated cases. The significant differences between the groups of patients with and without
swallowing disorders defined by the FEES were: the post-extubation time until the FEES,
the capacity to tolerate the FEES at upright sitting position (90°) vs. semi-upright sitting
position (60°), the abnormality of the Langmore scale and the abnormal movement of
the vocal cords. The complication registered in both groups was the presence of oxygen
saturation < 90%.
Conclusion: This study shows that the implementation of the FEES as a method for
detecting swallowing disorders (at the patient’s bedside) is safe.
Key words: Fiberoptic evaluation; Intensive care; Extubation
Introduction
Patients admitted to the critical care unit (CCU)
who require invasive mechanical ventilation
(IMV) will be exposed to laryngeal and tracheal
lesions, as a consequence of the admission cause
(example: serius injury) and also the presence
of the orotracheal tube, expressed as edema,
erythema or ulcers, among other lesions1,2. We
should also add the lesions caused by endoscopies,
tracheal aspirates, catheters and other procedures that may affect the patient’s swallowing, once he/she is extubated, in a transitory or even in a permanent manner.
The normal swallowing process is the coordinated action of a group of structures located in the
head, neck and thorax that implies a sequence of
events in which some functional sphincters open
to allow the progression of the bolus, transporting
it from the mouth to the esophagus, and then close
in order to avoid false paths and protect the airway.
This complex dynamic neuromuscular activity depends on a group of physiological behaviors
controlled by the activity of the central and peripheral nervous systems, causing the triggering
of the swallowing reflex3,4. The final objective of
this process is the nutrition of the individual. The
failure of this process is called dysphagia.
Dysphagia is a subjective feeling of difficulty in
making the food travel from the mouth to the stomach. The term dysphagia comes from two Greek
words, “dys” (difficulty) and “phagia” (eat). It
may be caused by an organic disorder or functional
difficulty and affects patients of all ages, from
babies to the elderly. Oropharyngeal dysphagia
includes swallowing disorders of oral, pharyngeal,
laryngeal and upper esophageal sphincter origin.
It involves almost 80% of diagnosed dysphagias.
It is a symptom that includes two important concepts: laryngeal penetration, involving the entry
of food up to the laryngeal vestibule, above the
level of the vocal cords, and aspiration, defined as
the entry of food to the larynx, below the level of
the vocal cords3,4.
The fiberoptic endoscopic evaluation of swallowing (FEES) is a technique that allows the study of
the physiology of swallowing, the estimation of risk
of aspiration, and guidance on the most secure way
to feed the patient in order to avoid complications
associated with swallowing disorders. It can be applied at the patient’s bedside, for approximately 20
minutes, by trained personnel, who can evaluate
various consistencies and progressive amounts of
different kinds of food, making this technique a
very attractive choice for the CCU, since it is not
necessary to transfer the patient to another place,
as for example the X-ray room, to carry out the
evaluation.
The objective of this study is the feasibility of the
FEES as a tool to evaluate swallowing at the CCU
and to know the incidence and types of swallowing
disorders at the CCU. To a lesser degree, we will
assess the Gugging Swallowing Screen5 (indirect
method for detecting swallowing disorders) using
the FEES for comparative purposes.
Materials and Methods
Design: comparative, prospective, analytical cohort
study conducted 24 hours after extubation for a
period of 6 months, including consecutively all
the patients who received mechanical ventilation for a period ≥ 48 hours. The enrollment began in
March, 2015.
The study was conducted at the CCU of the Hospital General de Agudos “Prof. Dr. Luis Güemes”,
Haedo, Buenos Aires. The hospital is a polyvalent
center of reference for referral of patients with
trauma and acute neurologic disease.
Primary Objective
1. Feasibility to carry out the FEES at the patient’s
bedside at the CCU.
2. Incidence of swallowing disorders in extubated
patients.
Secondary Objective
Also the GUSS (Gugging Swallowing Screen)5 will
be evaluated as a method for detecting swallowing
disorders in relation to the disorders found with
the FEES. The largest group of patients is the
one with neurologic diseases that would imply a
greater risk of suffering swallowing disorders, so
we will mainly analyze swallowing disorders in
that group. All the central nervous system events
(examples: ischemic or hemorrhagic stroke, subarachnoid hemorrhage, head trauma, convulsions,
central nervous system surgeries, etc.) were considered as neurologic diseases.
Sample Size
For a prospective cohort assuming an incidence of
38%, with 80% power and a 0.05 alpha level, the
N of patients to be included is 30.
Inclusion Criteria
Patients who required MV ≥ 48 hr and ≥ 24 hr
post-extubation.
Exclusion Criteria
Presence of delirium at the moment of the study
(evaluated with the CAM-ICU scale [Confusion
Assessment Method for the Intensive Care Unit])6;
pregnant women; limitation of therapeutic efforts;
basilar skull fracture; denial of the patient or his/her
family to participate in the study; tracheostomized
patients during this hospitalization or previous tracheal disease (patients with history of tracheotomy
present a higher probability of suffering swallowing
disorders); presence of facial trauma or any other
disease that prevents or contraindicates the insertion of the fribroscope through the nose.
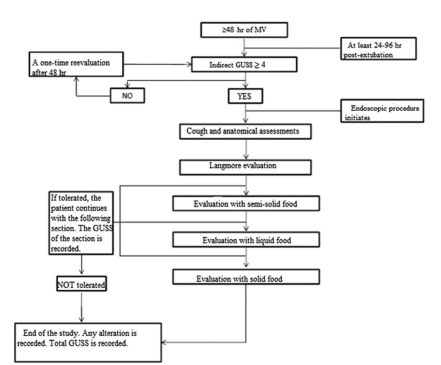
Evaluation Technique of the FEES
The evaluation of swallowing was carried out 24
hr after extubation, with a maximum period of 96
hr post-extubation. Before the FEES procedure,
we used the indirect GUSS (Gugging Swallowing
Screen) scale. In patients with an indirect GUSS
scale score ≥ 4, we carried out the FEES (Appendix
1 and 2)5. In patients with scores below 4, we reevaluated 48 hr later, in order to objectify whether
the indirect GUSS scale score had been modified
and meet the criteria for the FEES. If the score
was still below 4, the FEES wasn’t carried out in
that patient (Figure 1). The device we used was
an Olympus® BF Type P20D fibrobronchoscope
with 5 mm diameter, 2.2 mm working channel and
55 cm length.

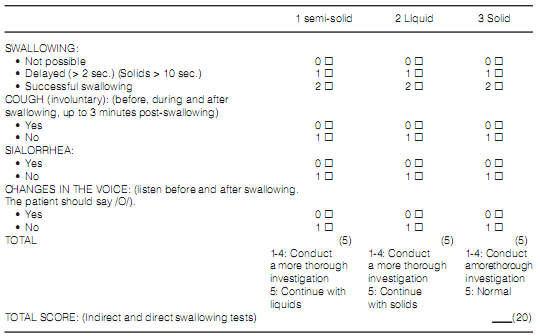
Description of the FEES technique (Figure 1): the patient was placed on the bed in an upright sitting position at 90°. If it was not possible, he/she was seated at 60°. Before inserting the device through one of the patient’s nostrils, we evaluated whether it was necessary to put up to 10 ml of lidocaine 2% gel in order to make the patient more comfortable (it does not affect the sensitivity of the study)7,8. Once the fibrobronchoscope was inserted, we continued until we could see the larynx. We recorded any anatomic alteration, stimulated the superior laryngeal nerve (aryepiglottic fold) in order to generate the cough reflex9 under stimulation and finally, we used the Basal Secretion Scale of Langmore (Appendix 3)3 in order to assess the management of salivary retention. Subsequently, always observing the larynx, we began with the intake of semi-solid foods (firm yoghurt with blue vegetal dye) in increasing concentrations (1/3 of a teaspoon, 1/2 tablespoon, and 1 tablespoon until reaching 5 tablespoons). If there wasn´t any procedure alteration, we proceeded with liquids and simultaneously recorded the GUSS scale according to the semi-solid food section. For the liquid food intake evaluation, we used increasing amounts of water with blue vegetable dye (3–5–10-20 cm3). If the patient tolerated the procedure, we continued with the evaluation of solid food intake, recording the GUSS scale score according to the liquid section. In the evaluation with solid foods we used a sufficient amount of bread crumb for the patient to form a bolus and try to swallow it. Like in the other stages of the procedure, we recorded the GUSS scale. Every stage (semi-solid/liquid/solid) had a maximum GUSS scale value of 5 points, with a total of 15 points in the direct evaluation, and a maximum of 5 points in the indirect evaluation (previously done), resulting in the final GUSS score1, 5. If the patient presents a swallowing disorder, the cause that motivated the end of the study by direct visualization must be recorded and qualified according to the modified Rosembeck Scale detailed below10, 11: 1) Subsequent effusion, which has to do with the presence of the food bolus at the hypopharynx (pyriform sinus) for more than 2 seconds before beginning the pharyngeal stage of swallowing; 2) Residues: persistence of food in the pharyngeal walls, pyriform sinus o valleculas after swallowing; 3) Laryngeal penetration: food entry to the laryngeal vestibule above the level of true vocal cords; 4) Aspiration: food goes beyond the level of true vocal cords, up to the trachea; 5) Reflux: food regurgitation from the esophagus, back to the larynx-pharynx. At the end of the study, the GUSS score must be recorded. In case of doubt about aspiration in one of the stages, the endoscope will proceed through the glottic area, going through the vocal cords, in order to evaluate whether there was aspiration or not.

During the procedure we looked for bronchospasms, presence of O2 saturation by pulse oximetry < 90%, nose bleeding, hypotension or any
other complication that could have arisen.
For the statistical analysis, continuous data are
expressed as mean value and standard deviation
or median and ranges, according to distribution.
Categorical data are expressed as frequency and
percentage. For the comparison of mean values, we used the Student’s Test or Mann-Whitney
Test, as applicable. For categorical data, we used
the Square Chi Test or Fisher’s Exact Test. We
carried out measures of association with Odds
Ratio and 95% confidence intervals. P values
below 0.05 of two-tailed test were considered as
significant.
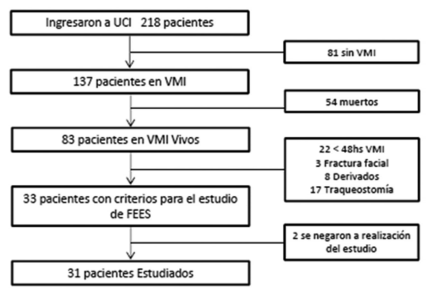
Results
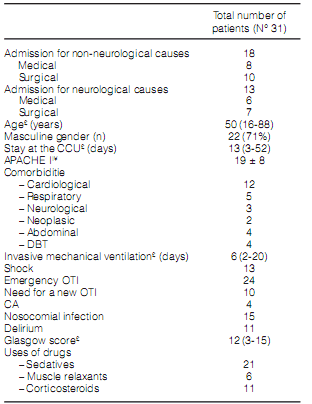
During the months of the study, 218 patients were admitted to the CCU. 31 patients were included in the protocol (Figure 2). The FEES could be completed in all the patients. The general characteristics of the patients are presented in Table 1.
The incidence of swallowing disorders in extubated patients who required MV was 58%, 95% CI
(0.407-0.735) with 18 cases of swallowing disorder
out of 31 evaluated cases.
Table 2 shows the characteristics of patients
with and without swallowing disorders, where the
only significant difference is the presence of shock
within the group with swallowing disorder.
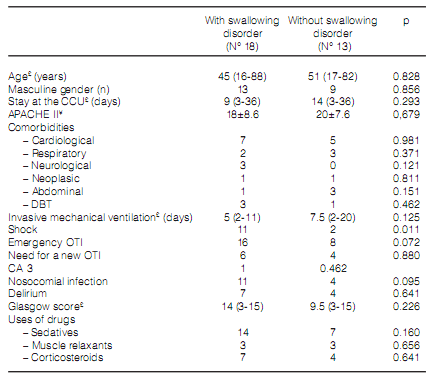
Table 3 shows the characteristics of these two groups of patients regarding the results of the FEES. We found significant differences in the postextubation time when we were doing the FEES, in the upright sitting position at 90° versus the semiupright sitting position at 60°, the abnormality in the Langmore scale and the abnormal movement of the vocal cords. The only complication we observed in both groups was the saturation < 90% that didn’t encourage the suspension of the study, for it was corrected in all the cases with supplementary O2, with no differences among patients with and without swallowing disorders. We did not observe bronchospasm, nose bleeding, hypotension nor any other complication.
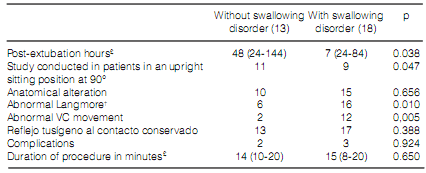
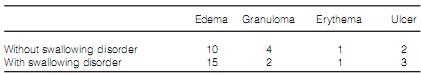
Tables 4 and 5 show the different anatomic alterations and the alterations by the Langmore scale found in patients, divided in groups.

The alterations observed according to the Rosembeck scale modified by the FEES in the 18 patients who presented alterations were: aspiration
in 8 patients, laryngeal penetration in 4 patients,
residues in 4 patients and esophageal reflux in 2
patients. The alterations were presented in the
following stages: before the evaluation with semisolid food, 4 patients; in the semi-solid food stage,
9 patients; in the liquid food stage, 5 patients, and
no alterations in the solid food stage.
Regarding the correlation between the FEES
and the GUSS, the presence of swallowing disorders by FEES has a significant correlation with a
GUSS score ≤ 14 (table 6), with a Kappa value of
0.867, p <0.001. The GUSS value ≤ 14 for detecting swallowing disorders has 100% sensitivity and
98% specificity.

The analysis of the subgroup of patients with
neurologic diagnosis shows a higher presence
of comorbidities, masculine gender and sensory
deterioriation (evaluated by the Glasgow score), whereas in the group of patients without neurologic diagnosis a greater use of sedatives (P 0.029)
was registered. The incidence of swallowing disorders wasn´t significant: 50% in the non-neurologic
group (9/18) and 69% in neurologic patients (9/13)
p 0.284.
Discussion:
MV is a treatment used in critically ill patients
for numerous causes. The consequent invasion of
the airway and anatomic sites where the orotracheal tube goes through exposes them to suffer
multiple lesions, anatomical and/or functional. For
example, the appearance of swallowing disorders
is significantly important, since it complicates
nutrition and consequently the patient’s rehabilitation. We should focus on the fact that the
impact of the swallowing disorder arises from a
complex interaction between the severity of the
clinical condition and the general condition of the
patient. That is why there are important variations
in the literature about the morbidity and mortality of swallowing disorders according to the study
population. Therefore, dysphagia, with potential
aspiration and its consequent pneumonia is one
of the most serious complications to be avoided in
this type of patients.
The frequency of dysphagia at the CCU
presents great variability depending on the patient’s history, the reason for admission and the
moment of the evaluation. Conservative opinions suggest that at least 20% of all extubated
patients who present respiratory insufficiency
could develop swallowing disorders12. Studies
with ≥ 48 hr of MV, such as the one of Barker et
al[13] showed that in patients with cardiac arrest,
there were 51% swallowing disorders (130 out of
254 patients), like the study of Ajemian et al14, which reported 56% of swallowing disorders
(27/48 patients). At the CCU, endoscopic evaluations of Leder et al15 showed 33% of swallowing
disorders in critically traumatized patients post
MV, and the evaluation of El Solh et al16 reported that 44% of the patients aspirated (37 out of 84
patients). They were elderly patients evaluated
by FEES who presented a critical condition and
required MV.
There aren’t numerous series in Argentina
regarding the incidence of swallowing disorders
in the post-extubation period, and there isn’t
much published experience in FEES at the CCU.
That is why we believe this study brings new
knowledge within a delicate topic not thoroughly
investigated in our country. The first important
fact is the incidence of swallowing disorders of
58%, which is higher than expected (38%). This
incidence is similar to previously cited studies of
other countries, taking into account the fact that
the patients evaluated in this study were critically ill, with hospitalizations of approximately 2 weeks
with MV, and were prematurely evaluated.
Patients who presented swallowing disorders
were evaluated first (median of 27 hr) in comparison with patients without swallowing disorders
(median of 48 hr) (see Table 3). This could be the
cause for such a high percentage, but we can’t discard the laryngeal dysfunction or edema as origin14.
Basing on this, we recommend in the future the
implementation of the FEES 48 hr post-extubation
in order to discard this group of patients.
Conducting the study in an upright sitting position at 90° seemed to have a protective effect, in
comparison with the position at 60°, though probably the patients who tolerated the 90° position
were the ones with a better general condition, since
that position mostly generated pain or discomfort
or the individual simply didn’t have the necessary
muscle tone to remain seated.
Aspiration is one of the main causes of in-patient
pneumonia, so it is important to know about it to
avoid complications. The consensus of “The North
American Summit on Aspiration in the Critically Ill
Patient”17 where aspiration is evaluated, estimates
that it is suffered by 45% of normal individuals during sleep, 70% of patients with impaired consciousness, from 0 to 40% of patients with enteral feeding
and between 50 and 75% of patients with MV. In our
study, 8 of the 18 patients with swallowing disorders
aspirated (44% of patients with disorders and 25%
of evaluated patients).
Both studies are important, but one of the advantages of the FEES, compared to the video swallowing exam is the possibility to observe anatomic
anomalies of the upper aerodigestive tract and
laryngeal lesions, very common in patients during
the post-extubation period. The study of Tadié et
al2 assessed the laryngeal anatomy of 136 patients.
Lesions were observed in 73% of the patients, with
the edema as the most common one, representing
59%. In the same study, the mobility of the vocal
cords was affected in 19% of the patients under
evaluation. In our work we detected 80% of anatomic anomalies (25/31 patients), with the edema
as the most common one, but not related to swallowing disorders. The abnormal mobility of the
vocal cords was important, with 45% compromise
(14/31 patients), and affected patients presented a
higher percentage of swallowing disorders.
Through direct visualization, in our study we
also used the Langmore scale. The alteration of the scale (with values > 0), was related to swallowing disorders. All the patients with values of
2 and 3 (which have to do with the accumulation
of secretions with overflowing that may dilute
at some moment or not, respectively) presented
swallowing disorders in 100% of the cases. That
is why a high score in the scale would predict a
swallowing disorder.
Regarding the comparison of the FEES with the
GUSS, we find certain values similar to those of the
study of Tralp M et al5, where a GUSS value ≤ 14
presented 100% sensitivity (just like our study) and
69% specificity (less than our study). This shows
that GUSS is a good predictor to assess swallowing
with the limitations of being an indirect method.
For more information about the clinical evaluation and decision making, we suggest the book of
Campora H et al, 2012 ed.4. We do not expand on
it due to limited space.
With reference to study limitations, the comparison with another method of similar value,
such as the video swallowing exam, is pending,
especially considering that many studies regard
it as the gold standard. Another limitation is the
number of patients evaluated for the analysis of
subgroups, such as neurologic patients. The study
was limited to a fixed time period. Probably, if
there were more patients under evaluation, the
tendency to have greater probabilities of suffering
swallowing disorders in neurologic patients could
result in a significant value.
Apart from the objective of this study, it provides
new information about the laryngeal pathology,
swallowing disorders and the implementation
of evaluation protocols post MV in order to feed
the patient safely and avoid complications during
spontaneous ventilation. It also provides information about an Argentinian center, our epidemiology and resources to solve problems based on the
limitations presented by us.
Conclusion
This study shows that the implementation of the FEES as a method for detecting swallowing disorders at the patient’s bedside is safe. There is high incidence of swallowing disorders in the postextubation period, affecting more than 50% of the evaluated patients. More studies are needed in order to determine in a reliable way that the FEES is the method for evaluating post-extubation swallowing.
Conflict of interest: this article was written with the Research Scholarship of the AAMR (Argentinian Association of Respiratory Medicine).
1. Radhakrishnan, S., U.K. Menon, and A. Anandakuttan, A combined approach of bedside clinical examination and flexible endoscopic evaluation of swallowing in poststroke dysphagia: A pilot study. Ann Indian Acad Neurol, 2013. 16(3): p. 388-93.
2. Tadie, J.M., et al., Post-intubation laryngeal injuries and extubation failure: a fiberoptic endoscopic study. Intensive Care Med, 2010. 36(6): p. 991-8.
3. Mª Mercedes Velasco, V.A., Pere Clavé, Carolina Puiggrós., Abordaje clínico de la disfagia orofaríngea: diagnóstico y tratamiento. Nutrición Clínica en Medicina, 2007. 1(3): p. 172 - 202.
4. Cámpora H, F.A., Evaluación y tratamiento de las alteraciones de la deglución. Re8v Am Med Resp, 2012. 3: p. 98 - 107.
5. Trapl, M., et al., Dysphagia bedside screening for acutestroke patients: the Gugging Swallowing Screen. Stroke, 2007. 38(11): p. 2948-52.
6. Tobar, E., et al., [Confusion Assessment Method for diagnosing delirium in ICU patients (CAM-ICU): cultural adaptation and validation of the Spanish version]. Med Intensiva, 2010. 34(1): p. 4-13.
7. Lester, S., et al., The effects of topical anesthetic on swallowing during nasoendoscopy. Laryngoscope, 2013. 123(7): p. 1704-8.
8. Kamarunas, E.E., et al., Effects of topical nasal anesthetic on fiberoptic endoscopic examination of swallowing with sensory testing (FEESST). Dysphagia, 2014. 29(1): p. 33-43.
9. Aviv, J.E., et al., The safety of flexible endoscopic evaluation of swallowing with sensory testing (FEESST): an analysis of 500 consecutive evaluations. Dysphagia, 2000. 15(1): p. 39-44.
10. Rosenbek, J.C., et al., A penetration-aspiration scale. Dysphagia, 1996. 11(2): p. 93-8.
11. Gonzalo Nazar M, A.O.T., Andrés Godoy M, José Miguel Godoy M, Inés Fuentealba M, Evaluación fibroscópica de la deglución. Revista de otorrinolaringología y cirugía de cabeza y cuello, 2008. 68(2): p. 131-142.
12. Skoretz, S.A., H.L. Flowers, and R. Martino, The incidence of dysphagia following endotracheal intubation: a systematic review. Chest, 2010. 137(3): p. 665-73.
13. Barker, J., et al., Incidence and impact of dysphagia in patients receiving prolonged endotracheal intubation after cardiac surgery. Can J Surg, 2009. 52(2): p. 119-24.
14. Ajemian, M.S., et al., Routine fiberoptic endoscopic evaluation of swallowing following prolonged intubation: implications for management. Arch Surg, 2001. 136(4): p. 434-7.
15. Leder, S.B., S.M. Cohn, and B.A. Moller, Fiberoptic endoscopic documentation of the high incidence of aspiration following extubation in critically ill trauma patients. Dysphagia, 1998. 13(4): p. 208-12.
16. El Solh, A., et al., Swallowing disorders post orotracheal intubation in the elderly. Intensive Care Med, 2003. 29(9): p. 1451-5.
17. McClave, S.A., et al., North American Summit on Aspiration in the Critically Ill Patient: consensus statement. JPEN J Parenter Enteral Nutr, 2002. 26(6 Suppl): p. S80-5.