Autor : Di Tullio Fernando1, Pascua Josefina1, Ernst Glenda1, Bosio MartĂn1, Salvado Alejandro1
1Department of Respiratory Medicine of the Hospital Británico
Correspondencia :
Abstract
Introduction: The purpose of this article is to describe the
characteristics, comorbidities and phenotypes of patients with
difficult-to-treat asthma (DTA) and severe asthma (SA).
Materials and Methods: Descriptive, cross-sectional study
of patients evaluated at the Difficult-to-Treat Asthma Clinic of the Hospital
Británico within the period of one year. We registered the age, gender
and anthropometric data, age of diagnosis, FEV1 at the beginning of follow-up
and previous exacerbations. We evaluated symptom control with the Asthma
Control Test and the Asthma Control Questionnaire. We registered the
comorbidities and evaluated the inflammatory profile of patients according to
blood biomarker measurements and induced sputum sample.
Results: Forty patients, 20 DTA and 20 SA. There weren’t
any significant differences regarding age, BMI, age of onset of symptoms,
symptom control or FEV1 at the beginning of follow-up. Crises were more common
in SA patients. The most commonly found coÂmorbidities were obesity, OSAHS and
gastroesophageal reflux disease. Psychiatric disorders were more common in SA
patients. The most commonly found phenotype was allergen-reactive TH2.
Discussion and Conclusion: it is not easy to classify both
groups, and many times there are overlapping characteristics. ComorÂbidities
are frequent in both groups: obesity, OSAHS and reflux disease are the most
common conditions. Being able to identify the asthma phenotype in order to
target the treatment.
Key words: Severe asthma; Difficult-to-treat asthma; Comorbidities
Received: 17-7-2021
Accepted: 29-10-2021
Introduction
Asthma is defined as a heterogeneous disease, usually
characterized by inflammation and chronic remodelling of the airways. It is
marked by sibilance, dyspnea, chest tightness and cough that varies with time
in terms of intensity, together with a limitation of the expiratory airflow.
Inflammation of the airways is associated with obstruction and bronchial
hyperresponse1.
Most asthma patients can be adequately treated with a combination of inhaled
corticosteroids (ICS) and bronchodilators, usually long-acting beta-adrenergic
agonists (LABA)2, 3.
However, there is a group of patients in whom it is difficult to adequately
control the symptoms, regardless of the indicated treatment. Taking into acÂcount
the reason for which the group can’t be controlled, the clinical management
guidelines define a subgroup as uncontrolled, difficult to control or
difficult-to-treat asthma (DTA) that has difficulties in controlling the
symptoms regardless of the medium or high doses of inhaled steroids and a
second follow-up controller. This lack of control could be due to the presence
of comorbidities, household or work exposure factors, disease refractoriness or
simply non-adherence to treatment or wrong use of inhaled devices1. Patients included in the
DTA group are those with severe asthma (SA), and without disease control,
despite the fact that they receive the complete treatment and show right
adherence and a suitable management of comorbidities1.
It is estimated that patients with difficult to treat asthma account for
approximately 17% of all asthmatic patients; and 3.7% are severe asthmatics,
representing 60% of asthma-related healthcare costs1,
4.
In the clinical practice it is difficult to make a categorical differentiation between patients with DTA and SA, since many comorbidities and exposure factors that would imply poor control of asthma are prevalent in the asthmatic population and are sometimes difficult to solve. It is common to find a diviÂsion between both groups only if symptoms get to be controlled. The purpose of this work is to describe the characteristics, comorbidities and phenotypes of patients with DTA and SA.
Materials
and Methods
Descriptive, cross-sectional study of patients evaluated at the
Difficult-to-Treat Asthma Clinic of the Hospital Británico from July
2018 to July 2019. The study included patients older than 18 years and those
who met the difficult-to-treat and severe asthma criteria according to the GINA
2019 guidelines1.
We registered age, gender and anthropometric data. We evaluated symptom control
with the Asthma Control Test (ACT) and Asthma Control Questionnaire (ACQ) in
the first consultation. Asthmatic exÂacerbation was defined as episodes
characterized by the worsening of the respiratory symptoms with increased
dyspnea, cough, sibilance or chest tightness and progressive loss of the
pulmonary function and those requiring some modification of the regular
treatment. It was defined as frequent exacerbation if there were ≥ 2 per
year and severe exacerbation if the patient required hospitalization1. We registered the
comorbidities including: obesity, patients with a body mass index (BMI) ≥
30 kg/m2, active smoking,
obstructive sleep apnea-hypopnea syndrome (OSAHS) defined by the presence of an
apnea-hypopnea index (AHI) ≥ 5, measured by respiratory polygraphy or
polysomnography in sleep laboratories5,
rhiÂnosinusitis and nasal polyps diagnosed by tomographic studies or previous
surgery. The diagnosis of gastroesophageal reflux disease (GERD) was
established by the presence of symptoms and/or when proven by digestive
endoscopy; and the history of psychiatric disorders was determined by the evaluÂation
carried out by the mental health team or by the presence of a previous
confirmed diagnosis. We evaluated the inflammatory profile of the patients
according to blood biomarker measurements, and in patients with SA we took
induced sputum samples, defining them as type 2 inflammation if they showed
blood eosinophilia ≥ 150/mm3 and/or eosinophil count ≥
2% in the sputum sample1, 4, 6, 7.
Patients not fulfilling this criteria with more than 40% neutrophil cell count
in the sputum sample were called neutrophilics7.
Also, the measurement of serum IgE > 100 UI/l was considered elevated. This
was found in patients with allergic asthma. We recorded the medication used in
both groups.
In the statistical analysis, results were presented as
percentages. For the numerical variables, results were shown as mean or
standard deviation (SD). The Mann-Whitney or Chi Square Tests were used to
compare differences between both groups. The results were analyzed with Prism 8
software (Graph Pad, La Jolla, CA).
Results
40 patients were included in the study for a period of one year,
20 of which met the criterion for difficult-to-treat asthma (DTA), and 20 for
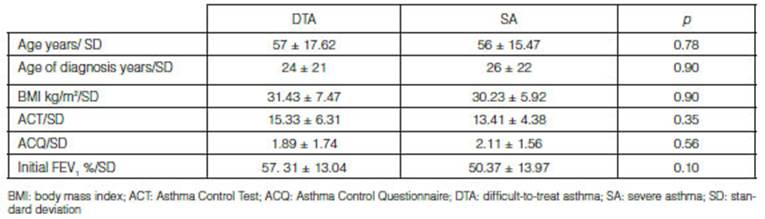
severe asthma (SA). The mean age was 57 ± 17.62 in DTA patients and 56 ± 15.47
years in SA patients. The mean BMI was 31.43 ± 7.47 and 30.23 ± 5.92 kg/m2
respectively, with no significant differences in any of the
variables. We discriminated the mean age of the asthma diagnosis in both
groups, which was 24 ± 21 years for the DTA group and 26 ± 22 for the SA group.
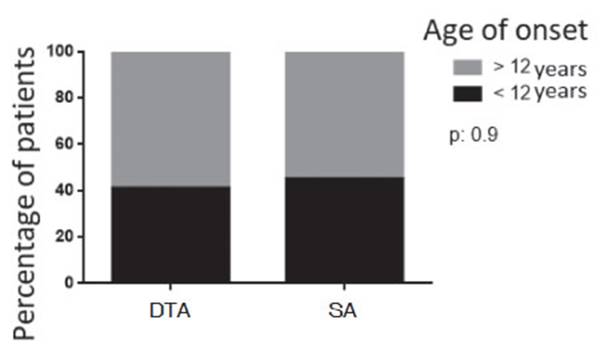
The diagnosis before 12 years of age accounted for 40% of the DTA patients and
45% of SA patients (p= 0.9) (Graphic 1). The mean result of the
ACT questionnaire at the beginning of the follow-up was 15.33 ± 6.31 in DTA and
13.41 ± 4.38 in SA (p = 0.35); and the ACQ results were 1.89 ± 1.74 and
2.11 ± 1.56, respectively (p = 0.5), no significant differences were
found. In the functional evaluation, the mean FEV1 percentage at the
beginning of the follow-up was 57 ± 13% in DTA and 50 ± 13% in SA (p =
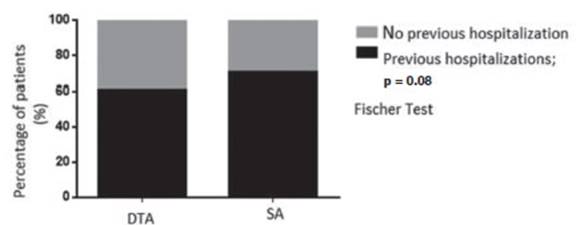
0.1) (Table 1). Seventy percent of SA and 60% of DTA had had previous
hospitalizations, non-significant difference were found (p = 0.08), (Graphic
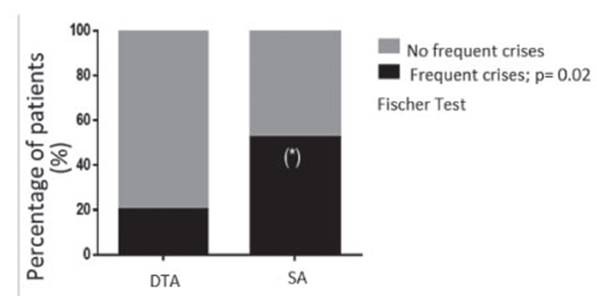
2). 55% of SA patients and 20% of DTA patients had frequent crises,
significant difference (p = 0.02) (Graphic 3). One hundred of
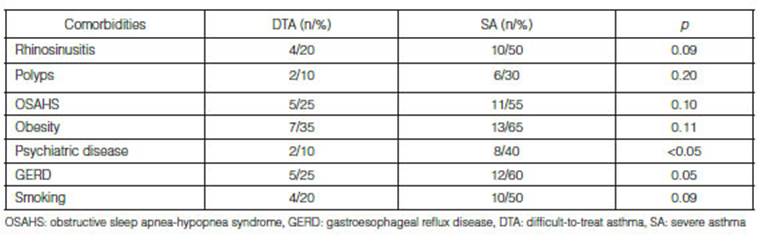
patients with SA and 85% of patients with DTA reported comorbidities; 2 out of
the 3 patients without comorbidities were overweight (BMI > 25 kg/m2). The most frequently
reported comorbidities in the SA group were: obesity, GERD and OSAHS; and in
the DTA group, the most frequent were GERD, obesity and OSAHS. History of
psychiatric disorders was more common in patients with SA (Table 2).
With regard to the biomarker study, 82% of the patients evaluated
in this study showed blood eoÂsinophilia ≥ 150/mm3,
90% in patients with SA and 50% in DTA, with a median of 639 (range between 34
and 1581) eosinophils /mm3 in SA and 271 (range between 53
and 5300) eosinophils/mm3 in DTA, non-significant
difference were found. Seventy seven percent of patients had an elevated IgE,
72% SA and 50% DTA, with a median of 233 (range between 9 and 1494) UI/l in SA
and 478 (range between 5 and 2229) UI/l in DTA, difference were found.
Differential cell count in sputum sample was performed in 19 patients with SA.
1 was eosinophilic, 2 neutrophilic and the rest were paucigranulocitic. In 3 paÂtients
with SA we detected IgE specific for Aspergillus, 2 of which met the criteria
for fungi-sensitized severe asthma8.
As for the treatment, the most commonly used combinations of
inhaled corticosteroids and bronchodiÂlators in 40 patients were
budenoside/formoterol, indicated in 57% of the cases, followed by fluticasone/
salmeterol, used in 30% of the cases; and the remaining patients received
fluticasone/vilanterol. 9 of the 20 SA patients received treatment with
biologicals. They all started with omalizumab; 3 patients changed to mepolizumab
due to a lack of response, and only one patient suspended treatment due to a
good clinical response. Omalizumab was indicated only in one patient from the
DTA group.
Discussion
Difficult -to-treat asthma accounts for approximately 17% of all
asthmatic patients, thus representing the highest healthcare cost within the
asthma spectrum. Comorbidities in these patients contribute to the existence of
a poor control of symptoms, but their exact impact on asthma control is not
fully established9.
In this work we found that comorbidities were common both in severe asthma
patients and in patients with difficult-to-treat asthma, mostly in the first
group, though the difference wasn’t significant. The most commonly found
comorbidity in both groups was obesity, which is possibly a factor that
contributes to poor asthma control and also to other comorbidities. Obese
patients with severe asthma show more exacerbations, worse control of symptoms,
greater use of oral steroids and alterations in functional evaluations10. There is 60% prevalence
of obesity in severe asthmatics11.
Obese patients belong to a determined phenotype and are most frequently
associated with a neutrophilic-type airway inflammatory profile, thus showing a
lower response to treatment with steroids12.
It has also been established that the lack of response could be mediated by a
defect in the glucocorticoid receptors and an increase in oxidative stress13. Obesity is also a risk
factor for developing other comorbidities such as OSAHS and GERD10,
which were very common in both groups. A mild weight loss that would imply
5-10% of the body weight is associated with better control of asthma symptoms14.
GERD is a risk factor for asthma exacerbation and poor control of
symptoms13,15. Asthmatic
patients have higher risk of developing GERD than the general population, with
a prevalence of 17 to 74%13,16,
in turn, patients with GERD have higher risk of having asthma compared to the
general population17.
Asthma is worse in patients with reflux, whether it is caused by a shifting
effect in airway hyperresponÂsiveness or due to the inflammation produced by
aspiration18.
Also the reflux may trigger symptoms of vocal cord dysfunction that can mimic
the asthma symptoms13.
With respect to the treatment of reflux in asthmatics, inconsistent results
have been obtained. Some studies showed an improvement in the symptoms, quality
of life and exacerbations related to the treatment of this comorbidity;
however, other studies weren’t able to prove this improvement19.
Asymptomatic patients are unlikely to be benefited from treatment with antacids13.
The asthma-OSAHS combination is associated with worse control of
respiratory symptoms, use of rescue short-lasting bronchodilators, rate of
exacerbations and lower quality of life20.
With OSAHS, asthma symptoms may increase, and asthma increases the risk of
developing OSAHS, regardless of obesity21.
Chronic rhinosinusitis, an asthma-related comorbidity, increases the risk of
suffering OSAHS22.
Sleep apneas increase the inflammation of the upper airway; and bronchial
neutrophilia and high levels of IL-8 have been reported in untreated patients
with OSAHS, compared to OSAHS patients who received treatment23.
In sleep apneas, the C-reactive protein, the TNF-α and cytokines involved in
systemic inflammation are elevated, regardless of the BMI, and could play a
determined role in the pathogenesis of asthma24.
Treatment with continuous positive airway pressure (CPAP) in asthmatic patients
improves asthma symptoms, reduces the use of bronchodilators, and improves the
peak espiratory flow and quality of life25,
and it has even been shown that in the first 7 days of treatÂment benefits
could already be observed26.
Anxiety and depression are psychiatric disorders found most
frequently in asthmatic patients than in the general population27.
These conditions are associated with lower adherence to treatment, difÂficulties
in follow-up and symptoms distortion. Patients with insomnia, anxiety and
depression have 2.4 times more possibilities of having poor control of
respiratory symptoms20.
It is recommended that during asthma follow-up an evaluation by trained
psychologists is carried out for the management of these patients28.
75% of asthmatic patients have symptoms of chronic sinusitis, and
the prevalence of this condition evaluated by tomography reaches up to 84% in
severe asthma patients29.
There could be a correlation between the level of inflammation of the upper
airways and the bronchi in patients with chronic sinusÂitis and severe asthma30. This comorbidity
associated with asthma is manifested with more coughing, expectoration and risk
of exacerbations15.
The presence of chronic rhinosinusitis associated with nasal polyps is
typically observed in patients with late onset asthma who may show allergy to
aspirin31.
Asthma is a heterogeneous condition. Asthma´s observable traits
(phenotypes), including the clinical characteristics of the disease and its
underlying mechanisms (endotypes) are complex and represent a multitude of
host-environment interactions. The cytology of the sputum provides evidence of
eosinophils, complex neutrophil mixed inflammation as well as few inflammatory
cells in some patients (pauciÂgranulocytics)32.
The T2-high endotype includes allergic asthma and late onset eosinophilic
asthma. Allergic asthma is characterized by an early onset, positive allergy
tests (skin or serum) with allergic rhinitis, IgE > 100 IU mL and mild
eosinophilia (< 300 μL). Eosinophilic asthma, on the other hand, is
characterized by a late onset, negative allergy tests; low IgE, nasal
poly-posis and eosinophilia (300 blood eosinophils/mm3 or > 2% sputum eosinophils)33. 70% of evaluated
patients with SA had more than 300 eosinophils/mm3,
and most patients were allergic, given that 74% had an elevated IgE. The
correct phenotipification of the DTA patient, and specially patients diagnosed
with SA would allow a targeted treatment.
Conclusions
The anthropometric characteristics, control of symptoms and FEV1
in patients with SA and DTA were similar. During follow-up, comorbidities were
detected frequently in both groups, especially obesity, GERD and sleep apneas,
which are interconnected. Though they were more common in the SA group, there
weren’t any significant differences between both groups. The history of
psychiatric diseases was more common in the group of SA. There were significant
differences regarding frequent exacerbations, which were stronger in patients
with SA.
It isn’t easy to classify both groups, and most patients show
overlapping characteristics. ComorbidiÂties occur frequently in both groups,
and many of them are difficult to treat or need a long time to be resolved or
controlled, such as obesity, rhinosinusitis or smoking; however, with the
biomarker analysis and clinical history, the swelling agent is still in many
cases the main target of these patients’ treatÂment. So, in patients with
difficult-to-treat asthma, we should identify the phenotype of the disease,
because even with unresolved comorbidities, the progression of the asthma treatment
shouldn’t be limited, especially in cases of inadequate disease control.
References
1. Global Initiative for Asthma. GINA 2019. Glob. Strateg. Asthma
Manag. Prevention (2019).
2. Carlstrom, L. & Castro, M. Severe asthma: What makes it so hard to manage? Current Allergy and Asthma
Reports 2009; 9(5): 393-400.
3. Lemière C, Pierre E, Ron O, et al. Airway inflammation
assessed by invasive and noninvasive means in severe asthma: Eosinophilic and
noneosinophilic phenotypes. J. Allergy Clin. Immunol. 2006; 118(5): 1033-9.
4. Israel, E. & Reddel, H. K. Severe and difficult-to-treat
asthma in adults. New England Journal of Medicine. 2017; 377(10): 965-76.
5. Nogueira F, Borsini E, Cambursano H, et al. Guías
prácticas de diagnóstico y tratamiento del síndrome de
apneas e hipopneas obstructivas del sueño. Medicina. 2013; 19: 59-90.
6. Wenzel SE. Asthma phenotypes: The evolution from clinical to
molecular approaches. Nat Med. 2012; 18: 716-25.
7. Moore WC, Annette TH, Xingnan L, et al. Sputum neutrophil
counts are associated with more severe asthma phenotypes using cluster
analysis. J. Allergy Clin. Immunol. 2014; 133(6): 1557-63.
8. Agarwal R. Severe asthma with fungal sensitization. Curr Allergy
Asthma Rep. 2011; 11: 403-13.
9. Bisaccioni C, Vivolo M, Cajuela E, et al. Comorbidities in
severe asthma: Frequency of rhinitis, nasal polyposis, gastroesophaÂgeal reflux
disease, vocal cord dysfunction and bronchiectasis. Clinics 2009; 64(8): 769-73.
10. Gibeon D, Batuwita K, Osmond M, et al. Obesity-associated
severe asthma represents a distinct clinical phenotype analysis of the british
thoracic society difficult asthma registry patient cohort according to bmi.
Chest 2013; 143(2): 406-14.
11. Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy
Clin Immunol 2018; 141(4): 1169-79.
12. Sutherland ER, Goleva E, Strand M, et al. Body mass and
glucocorticoid response in asthma. Am. J. Respir. Crit Care Med. 2008; 178(7):
682-7.
13. Porsbjerg C, Menzies-Gow A. Comorbidities in severe asthma:
Clinical impact and management. Respirology 2017; 22(4): 651-61.
14. Scott H, Gibson P, Garg M, et al. Dietary restriction and
exercise improve airway inflammation and clinical outcomes in overweight and
obese asthma: A randomized trial. Clin Exp. Allergy 2013; 43(1): 36-49.
15. Tay TR, Radhakrishna N, Hore-Lacy F, et al. Comorbidities in
difficult asthma are independent risk factors for frequent exacerbations, poor
control and diminished quality of life. Respirology 2016; 21(8): 1384-90.
16. Sontag SJ, O’Connell S, Khandelwal S, et al. Asthmatics with
gastroesophageal reflux: Long term results of a randomized trial of medical and
surgical antireflux therapies. Am J Gastroenterol. 2003;98(5): 987-99.
17. Tsai MC, Lin HL, Lin CC, et al. Increased risk of concurrent
asthma among patients with gastroesophageal reflux disease: A nationwide
population-based study. Eur J Gastroenterol. Hepatol. 2010; 22(10): 1169-73.
18. McCallister JW, Parsons JP, Mastronarde JG. The relationship
between gastroesophageal reflux and asthma: An update. Therapeutic Advances in
Respiratory Disease 2011; 5(2): 143-50.
19. Gibson PG, Henry R, Coughlan JJ. Gastro-oesophageal reflux
treatment for asthma in adults and children. Cochrane DataÂbase Syst. Rev.
2003; (2): CD001496.
20. Teodorescu M, Broytman O, Curran-Everett D, et al. Obstructive
sleep apnea risk, asthma burden, and lower airway inflamÂmation in adults in
the severe asthma research program (SARP) II. J Allergy Clin. Immunol Pract.
2015; 3(4): 566-75.
21. Rogers L. Role of Sleep Apnea and Gastroesophageal Reflux in
Severe Asthma. Immunol Allergy Clin of North Am 2016; 36(3): 461-71.
22. Jiang RS, Liang KL, Hsin CH, Su MC. The impact of chronic
rhinosinusitis on sleep-disordered breathing. Rhinology. 2016; 54(1): 75-9.
23. Devouassoux G, Lévy P, Rossini E, et al. Sleep apnea is
associated with bronchial inflammation and continuous positive airway
pressure-induced airway hyperresponsiveness. J. Allergy Clin Immunol. 2007; 119(3):
597-603.
24. Ciftci TU, Kokturk O, Bukan, N, Bilgihan A. The relationship
between serum cytokine levels with obesity and obstructive sleep apnea
syndrome. Cytokine 2004; 28(2): 87-91.
25. Lafond C, Sériès F, Lemière C. Impact of
CPAP on asthmatic patients with obstructive sleep apnoea. Eur Respir J. 2007;
29(2): 307-11.
26. Busk M, Busk N, Puntenney P, et al. Use of continuous positive
airway pressure reduces airway reactivity in adults with asthma. Eur Respir J.
2013; 41(2): 317-22.
27. Lavoie KL, Bacon S, Silvana B, et al. What is worse for asthma
control and quality of life: Depressive disorders, anxiety
disorders, or both? Chest 2006; 130(4): 1039-47.
28. McDonald VM, Vertigan AE, Gibson PG. How to set up a severe
asthma service. Respirology. 2011; 16(6): 900-11.
29. Ten Brinke A, Grootendorst D, Schmidt J, et al. Chronic
sinusitis in severe asthma is related to sputum eosinophilia. J AlÂlergy Clin.
Immunol. 2002;109(4): 621-6.
30. Ten Brinke, A. Sterk, P. Masclee, A. et al. Risk factors of frequent
exacerbations in difficult-to-treat asthma. Eur Respir J. 2005;27(6): 1324-5.
31. Kowalski ML, Asero R, Bavbek S, et al. Classification and
practical approach to the diagnosis and management of hypersenÂsitivity to
nonsteroidal anti-inflammatory drugs. Allergy EurJ Allergy Clin Immunol. 2013;
68(10): 1219-32.
32. Papi A, Brightling C, Pedersen SE, Reddel HK. Seminar: Asthma.
Lancet. 2018; 391: 783-800.
33. López Viña A. Solapamiento en el asma grave T2:
Âżhacia dónde se inclina la balanza? Rev Patol Resp. 2019; 22: 141-2.