Autor : Bastidas Goyes Alirio Rodrigo1,2,3 Afanador Ardila Juan Sebastián2,3 Bueno LĂłpez Jorge Eduardo2,3 Parra Charris Andes Eduardo2,3 PinzĂłn Saavedra Andrea Catalina2,3 Barragán Amado AndrĂ©s Felipe2,3 MartĂn Arsanios Daniel Augusto2,3
1Department of Respiratory Medicine, ClĂnica Universidad de la Sabana, Cundinamarca, ChĂa, Colombia 2Faculty of Medicine, Universidad de la Sabana, Cundinamarca, ChĂa, Colombia 3Research Unit, Faculty of Medicine of the Universidad de la Sabana, ChĂa, Cundinamarca, Colombia Institution: Universidad de la Sabana, ChĂa, Cundinamarca, Colombia. Campus del Puente del ComĂşn, Km. 7, Autopista Norte, Bogotá. Zip Code: 250001
Correspondencia :Alirio R. Bastidas Goyes. Email: alirio.bastidas@unisabana.edu.co
Abstract
Introduction: Chronic obstructive pulmonary disease is a
condition with high prevalence worldwide. It is preventable and treatable, but
with very high levels of underdiagnosis. The use of screening tools is
imperative. These tools are easily applied, interpreted, and validated in
different populations and help not only the clinician to confirm the diagnostic
suspicion, but also the patients to become aware of their disease. The
objective is to validate the COPD-Population Screener Questionnaire (COPD-PS)
in one Colombian population.
Materials and Methods: A prospective cohort study was
carried out. Participants had to be older than 40 years, show a good quality
spirometry, and have completed the COPD-PS questionnaire twice. COPD was
defined as FEV1/FVC < 0.7 and with a history of exposure to tobacco smoke. A
reproducibility and validation analysis has been conducted.
Result: Out of a total of 2.199 potential subjects, 1.662 entered the
final analysis; the prevalence of COPD in the study was 21.1%. With the COPD-PS
questionnaire cut-off point of four, the sensitivity was 77.2% and the
specificity was 46.3%, with an area under the receiver operating characteristic
curve of: 0.66 (95% CI: 0.63-0.69) (p<0.01). An intraclass correlation
coefficient of 0.817 (95% CI: 0.79-0.84) and a kappa coefficient of: 0.45 (95%
CI: 0.31-0.59) (p<0.01) were obtained.
Conclusion: The COPD-PS questionnaire is a tool with high
sensitivity and good reproducibility for the screening of COPD, and could
suggest the use of a spirometry in subjects not diagnosed with this disease.
Key words: Chronic obstructive pulmonary disease; Questionnaire;
Reproducibility of results; Lung function test.
Received: 01/18/2021
Accepted: 08/12/2021
Introduction
Chronic obstructive pulmonary disease (COPD) is the fourth leading
cause of mortality throughout the world. It is estimated that it affects more
than 5 percent of the world population1,
and 8-10% people older than 40 years2-4.
However, despite the fact of being a very common and well-known disease in the
medical practice, it is highly underdiagnosed. The reason for this
underdiagnosis is that patients do not attend their consultation because they
have become used to the symptoms or they don’t know for sure if COPD can be
treated. Also, the spirometry isn’t easily interpreted by some medical care providers
and sometimes COPD is mistaken for asthma, because both conditions may show
fixed airflow obstruction5-7.
One of the questionnaires to be used was the Chronic Obstructive
Pulmonary Disease Population Screener (COPD-PS)5.
It consists of five items: three items related to symptoms (dyspnea, producÂtive
cough and activity limitation), a fourth item related to smoking history (100
or more cigarettes smoked throughout the patient’s life) and the last item
associated with age. It has a maximum score of 10 points10.
In its initial validation study done in the United States in 2008, with a
cut-off point ≥ 5, it showed 84.4% sensitivity and 60.7% specificity.
Then it was validated in 2021 and 2014 in Spain and Japan with a cut-off point
of 4, and obtained the following results: 93.6% sensitivity and 64.8% specificity
in the Iberian country.
In Colombia, COPD is a prevalent disease, where the medical record
or the use of screening questionÂnaires may be useful for searching patients
with this disease4, 15, 16;
however, in order to use screening instruments, it is necessary first to
conduct validation studies ideally in the populations where they are intended
to be used. In the country there is lack of data about the diagnostic
performance of specific questionnaires such as the COPD-PS. The objective of
this study is to determine the reproducibility and validation of the COPD-PS
questionnaire in a Colombian population.
Methodology
We carried out a prospective cohort with the objective of
determining the reproducibility and validaÂtion of the COPD-PS questionnaire in
a Colombian population. The participants attended an external consultation at
the Clínica Universidad de la Sabana, Chía, Colombia. Patients
were enrolled between 2015-2020.
Population
Individuals older than 40 years who were asked to undergo a spirometry
to be performed in the lung function laboratory of a third level clinic,
regardless of their indication. Individuals needed to have enough time
available for this study and had to give their consent to answer a lung
function questionÂnaire. We excluded subjects whose spirometry didn’t fulfill
the acceptability or reproducibility criteria of the American Thoracic Society
(ATS) guidelines, or subjects with some kind of limitation on their
communication that would complicate the development of clinical questionnaires.
The spirometry was performed by the duly qualified and trained staff of the
lung function laboratory, with previously caliÂbrated equipment. COPD was
defined as the presence of fixed airflow obstruction with a FEV1/FVC ratio of
less than 0.7 after the administration of the bronchodilator, according to the
ATS definition, and with a smoking history of more than 10 packs per year.
Study
variables and data gathering
Data gathering included a first visit to obtain demographic
information: age, sex, race, weight, height, level of education, respiratory
symptoms, history of exposure to tobacco smoke, wood smoke or other
occupational exposure to smoke, history of medical diagnosis of COPD or asthma
(confirmed or not confirmed) and lung function values. The COPD-PS
questionnaire was applied at the end of the spiÂrometry and subsequently during
a second visit with a minimum difference of 15 days between them, where the
questionnaire was repeated for the reproducibility analysis.
COPD-PS
questionnaire
The Spanish version of the COPD-PS questionnaire consists of the
following 5 questions: 1. For the past 4 weeks, how many times did you feel
breathless? 2. Do you sometimes expel something such as mucus or sputum when
you cough? 3. During the last year, did you reduce your daily activities due to
your respiratory problems? 4. Did you smoke at least 100 cigarettes throughout
your life? 5. How old are you? Each question has multiple answers with a score
that goes from 0 to 2, with a maximum total score of 10 points. A value ≥
4 is considered high risk of having chronic obstructive pulmonary disease
(COPD) and need to do additional tests.
Sample size
In order to calculate the sample size, we used data from the
studies of Martínez FJ, which showed 84.4% sensitivity and 60.7%
specificity (5) for the COPD-PS questionnaire; also, the studies of Miravitles
M, who reported 93.6% sensitivity and 64.8% specificity10,
and of Tsukuya G, who found 67.1% sensitivity and 72.9% specificity for the
same questionnaire11.
With this information, for a COPD prevalence of 8.9%4,
95% confidence level and 2% precision we required a minimum of 2012 subjects.
The subjects entered the study in a sequential manner, and those who didn’t
fulfill the inclusion criteria were reÂplaced by others until the sample size
was exceeded.
Data
analysis
Data were obtained through the REDCap platform (web platform for
building and managing online surveys and databases), and then they were
analyzed with the statistical program SPSS, version 25. An initial description
of qualitative variables was made in frequencies and percentages, and a
description of quantitative variables was carried out using mean and standard
deviation if their distribution was normal, or median and interquartile range
if it wasn’t. Then, we compared and analyzed quantitative variables through the
Student T Test or the Mann-Withney U Test according to their distribution and
also compared qualitative variables through the Chi-Square Test. We calculated
the sensitivity, specificÂity, positive predictive value (PPV), negative
predictive value (NPV), positive likelihood ratio (LR+), negative likelihood
ratio (LR-), number needed to screen and number needed to harm. To evaluate
reproducibility, we calculated the kappa coefficient and the intraclass
correlation coefficient (ICC). Finally, we calculated the area under the
receiver operating characteristic curve (AUROC) with the values of the COPD-PS
questionnaire. We estimated the 95% confidence intervals for the reproducÂibility
measures obtained and considered p <0.05 as statistically significant.
Ethical
considerations
The research protocol followed the international ethical
guidelines of the Declaration of Helsinki, the country’s ethical considerations
of the 8430 resolution of 1993 and the Data Protection Law 1581. It was
presented and approved by the Research Committee of the Universidad de la
Sabana and by the Ethics Committee of the Clínica Universidad de La
Sabana.
Results
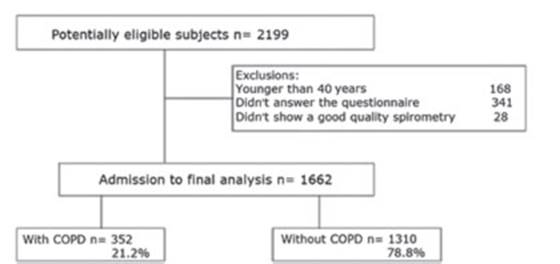
Of a total of 2199 potentially eligible subjects for the study,
1662 participated in the final analysis. Figure 1 shows the flow of
subjects through admission to the study and the respective exclusions.
General
characteristics of the population
The mean age was 70.04 years (SD: 10.8); 86.1% were mixed-race;
56.8% were males and there was a COPD prevalence of 21.2%. 89.04% had indicated
some respiratory symptom and 51.9% of the popuÂlation had completed basic
primary education. Table 1 shows the characteristics of the population,
respiratory symptoms, their background and the results of the lung function
tests.
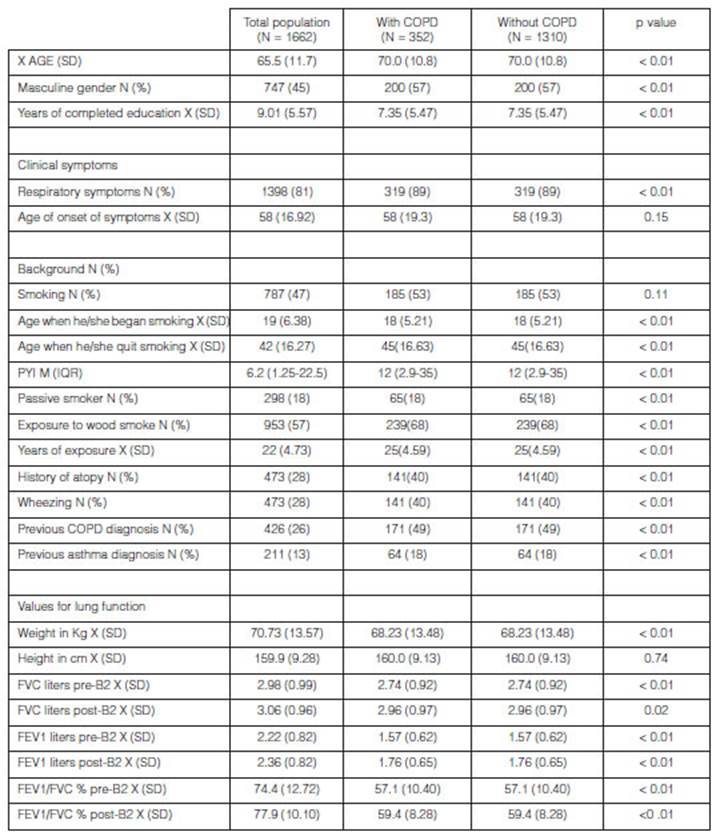
COPD:
chronic obstructive pulmonary disease; PYI: packs-year index; X: average; SD:
standard deviation; N: number or frequency; FVC: forced vital capacity; FEV1:
forced expiratory volume in the first second.
Results of
the COPDS-PS questionnaire
Table 2 shows the answers to the COPD-PS questionnaire, classified by each
one of the questions and with the total score, both in subjects with and
without COPD. It is evidenced that each question and the average score of the
questionnaires in both groups have a statistically significant p value for the
diagnosis of COPD. The average response time for the questionnaire was
calculated at 1 minute.
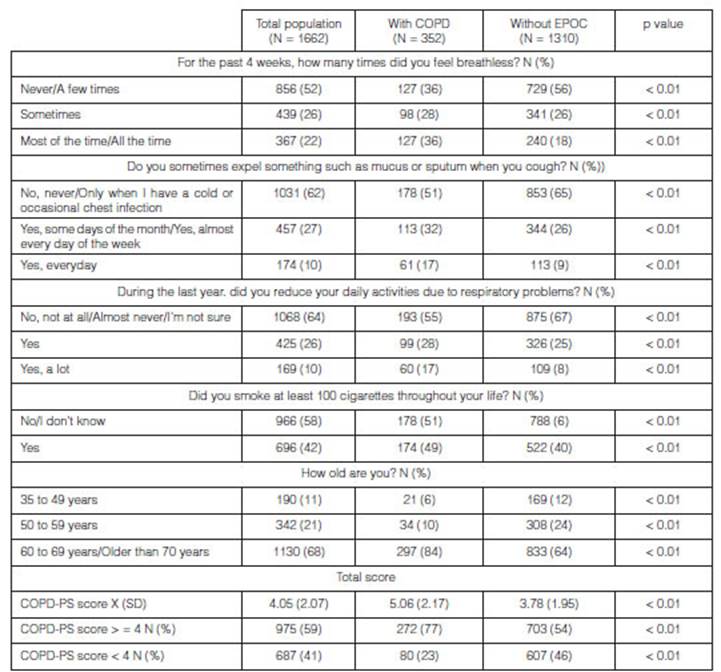
X: average;
SD: standard deviation; N: number or frequency.
Reproducibility
of results and validation of the COPD/PS questionnaire
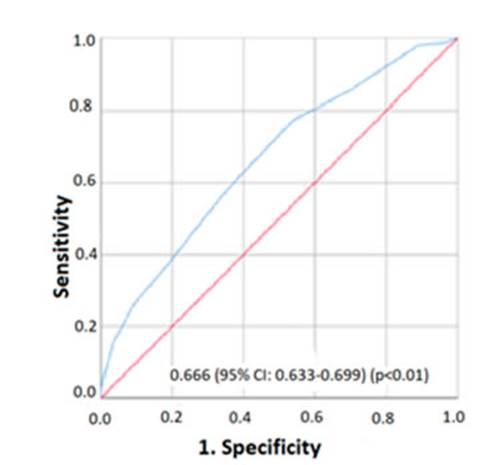
Table 3 shows the analysis of sensitivity and specificity characteristics
of the COPD-PS questionnaire at different cut-off points. The Youden index that
determined the highest sensitivity together with the specificity (0.77-0.46,
respectively) used a cut-off point ≥ 4. Also, for that cut-off point we found
a posiÂtive predictive value of 2.279 and a negative predictive value of 0.883,
and a positive likelihood ratio of 1.439 and a negative likelihood ratio of
0.490.
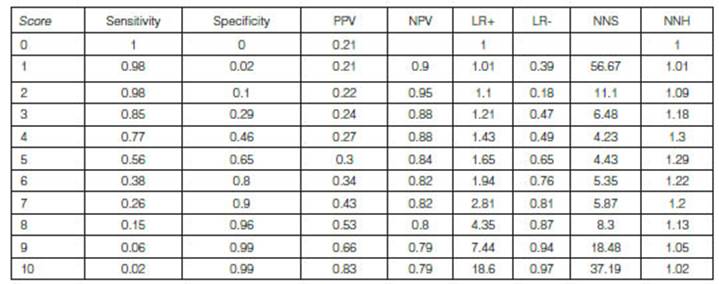
PPV:
positive predictive value; NPV: negative predictive value; LR +: positive
likelihood ratio; LR -: negative likelihood ratio; NNS: number necessary to
screen; NNH: number necessary to harm.
Discussion
In this study we evaluate the validation of the COPD-PS
questionnaire for the screening of COPD in a Latin American population; the
performance is good when compared to the value of the FEV1/FVC ratio of less
than 0.7 post- bronchodilator (AUROC:0.666 95% CI:0.633-0.699). This
performance was similar to that found by Tsukuya G11,
who found an AUROC of 0.74 in the healthy overall population; however, with
this score, AUROC values of even 0.88 have been found and reported by
Miravitlles M10 in a population of
patients at the first level of care. The sensitivity in the last study reached
93.6%; that is 16.4 points higher than the sensitivity observed in our study.
Such difference may be due to a higher degree of exposure to smoking in the
Spanish population studied by Miravitlles M et al., where the mean PYI was
42.79, twice as much as the one found in our study (PYI: 22).
The specificity found in our study (46.3%) is the lowest, compared
to the validation studies of the COPD-PS questionnaire mentioned before; given
the fact that this questionnaire evaluates respiratory symptoms, its capacity
depends on the expression of such symptoms. In our population, the respiratory
symptoms may eventually be expressed in a different way, maybe due to the high
frequency of chronic bronchitis (CB) and the influence of the altitude.
Subjects with CB can have mild obstruction not exÂpressing tobacco exposure
exclusively, thus affecting this score17,
18. On the other hand, Horner found that subjects who live
in geographical areas higher than 1500 MASL show less respiratory symptoms,
even the ones with COPD19.
We shall remember that when doing screening tests, we expect sensitivity to be
higher than specificity.
Other validations prior to our study used two cut-off points. In
the first validation analysis conducted in the United States a cut-off point of
five was used. It reported adequate sensitivity and specificity values4. But subsequent
validation analyses conducted in Spanish and Japanese populations used a
cut-off point of four, also used in our study, because with that number a
better sensitivity can be obÂtained, with an acceptable reduction in the
specificity of these populations10, 11.
The reason for these differences is not clear, but the cut-off points of
different diagnostic tests may vary depending on the characteristics of the
populations being evaluated, hence the importance of conducting the respective
validation analyses. In our study, the COPD-PS questionnaire showed excellent
test-retest reproducÂibility and reliability, with very good intraclass
correlation coefficients and kappa coefficients for the dichotomous responses,
both in the overall population and in the COPD and non-COPD groups. This favors
the use of this tool.
In our study, the prevalence of COPD was 21.1%, similar to that
reported in the PUMA study in a hospital environment; and obviously higher than
the one reported in a general Colombian and Latin American population. The
PREPOCOL study carried out in a community-based population showed that 9 out of
100 people older than 40 years had COPD, setting a prevalence of 8.9%4, 20, 21. It is known that
a higher prevalence affects the positive predictive value and limits the
extrapolation of the study results to the overall population; however, this
questionnaire may be useful for the medical evaluation of different care levels
where COPD underdiagnosis rates are still high, as shown by a study conducted
in Argentina which found an underdiagnosis rate of 77.4%. And, in the PLATINO
study the rate was 88.7%19, 22-24.
To make the diagnosis of COPD, it is necessary to have tools that
are easy to use, access and underÂstand by the overall population, that can be
used for case detection or screening both in the community and in the hospital
environment, and that aren’t used exclusively by professionals specialized in
internal medicine and pulmonology. The objective of having such tools is to
raise awareness among patients about their health status but also to create an
alert in the healthcare personnel that makes them do tests such as a spirometry
for early diagnosis, and take preventive measures such as quitting smoking and
timely treatment. Despite its validation, reproducibility and potential
benefits as a detection test for COPD patients in our population, some
weaknesses should also be taken into account: the type of population of the
study, which may limit the extrapolation of results, and the lack of cost
estimates with which the findings of this study could have been strengthened.
In future studies in our population, we could include in the evaluation of
these scores the assessment of risk factors such as exposure to wood smoke and
the estimation of the economic impact upon the use of these tools.
Conclusion
The COPD-PS questionnaire is a tool with high sensitivity and good
reproducibility for the screening of COPD, and could suggest the use of a
spirometry in subjects not diagnosed with this disease.
Conflicts of interest
None.
Funding
This study hasn’t received any financial support or scholarship
from public or private sectors. It is a non-profit study.
Ethical responsibilities
Protection of people and animals: The authors declare that for
this research no experiments have been done on human beings or animals.
Data confidentiality: The authors declare they have followed the protocols
of their work center on the publication of patient’s data.
Right to privacy and informed consent. The authors declare this
article doesn’t provide any informaÂtion about the patients.
References
1. Kochanek KD, Murphy S, Xu J, Arias E. Mortality in the United
States, 2016. NCHS Data Brief. 2017; (293): 1-8.
2. Mathers CD, Loncar D. Projections of global mortality and
burden of disease from 2002 to 2030. PLoS Med. 2006; 3(11): e442.
3. Kaplan A, Thomas M. Screening for COPD: the gap between logic
and evidence. Eur Respir Rev. 2017; 26(143):160113.
4. Caballero A, Torres-Duque CA, Jaramillo C, et al. Prevalence of
COPD in five Colombian cities situated at low, medium, and high altitude
(PREPOCOL study). Chest. 2008; 133(2): 343-9.
5. Martinez FJ, Raczek AE, Seifer FD, et al. Development and
initial validation of a self-scored COPD Population Screener Questionnaire
(COPD-PS). COPD. 2008; 5(2): 85-95.
6. GOLD-2020-REPORT-ver1.0wms.pdf [Internet]. [cited on July 17
2020]. Available at: https://goldcopd.org/wp-content/ uploads/2019/11/GOLD-2020-
REPORT-ver1.0wms.pdf
7. Albers F, Shaikh A, Iqbal A. Design, rationale, and baseline
demographics of SEARCH I: a prospective cluster-randomized study. Int J Chron
Obstruct Pulmon Dis. 2012; 7: 437-45.
8. de GesEPOC G de T. Proceso de la atención inicial al
paciente con EPOC. Estrategias de cribado. Arch Bronconeumol. 2017; 53: 15-21.
9. Sui CF, Ming LC, Neoh CF, Ibrahim B. VitalQPlus: a potential
screening tool for early diagnosis of COPD. Int J Chron ObÂstruct Pulmon Dis
[Internet]. 2015; 10: 1613-22.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4541542/
10. Miravitlles M, Llor C, Calvo E, Diaz S, Diaz-Cuervo H, Gonzalez-Rojas
N. [Validation of the Spanish version of the Chronic Obstructive Pulmonary
Disease- Population Screener (COPD-PS). Its usefulness and that of FEV(1)/FEV(6) for the diagnosis of COPD]. Med Clin Barc. 2012;
139(12): 522-30.
11. Tsukuya G, Matsumoto K, Fukuyama S, et al. Validation of a
COPD screening questionnaire and establishment of diagnostic cut-points in a
Japanese general population: the Hisayama study. Allergol Int. 2015; 64(1):
49-53.
12. Chung KS, Jung JY, Park MS, et al. Cut-off value of FEV1/FEV6
as a surrogate for FEV1/FVC for detecting airway obstruction in a Korean
population [Internet]. International Journal of Chronic Obstructive Pulmonary
Disease. 2016 [cited on July 17 2020].
https://www.dovepress.com/cut-off-value-of-fev1fev6-as-a-surrogate-for-fev1fvc-
for-detecting-air-peer-reviewed-full-text-article-COPD
13. Kobayashi S, Hanagama M, Yanai M. Early Detection of Chronic
Obstructive Pulmonary Disease in Primary Care. Intern Med [Internet]. 2017;
56(23): 3153-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5742385/
14. Hidalgo Sierra V, Hernández Mezquita M, Palomo Cobos L,
et al. Usefulness of The Piko-6 Portable Device for Early COPD Detection in
Primary Care. Arch Bronconeumol. 2018; 54(9): 460-6.
15. Londoño D, García OM, Celis C, et al.
Guía de práctica clínica basada en la evidencia para la
prevención, diagnóstico, trataÂmiento y seguimiento de la
enfermedad pulmonar obstructiva crónica (EPOC) en población
adulta. Acta Médica Colomb. 2014; 39(2 SI (2)): 5-49.
16. Bernal Pinilla J. Carga de la enfermedad pulmonar obstructiva
crónica en Colombia. 2015 [cited on July 17 2020]; http://repository.javeriana.edu.co/handle/10554/19457386
387
Validation and reproducibility of the COPD-PS questionnaire for
the screening of COPD
17. Torres-Duque CA, García-Rodriguez MC,
González-García M. Is Chronic Obstructive Pulmonary Disease
Caused by Wood Smoke a Different Phenotype or a Different Entity? Arch
Bronconeumol Engl Ed [Internet]. 2016; 52(8): 425-31. Available at:
http://www.sciencedirect.com/science/article/pii/S1579212916301306
18. Gonzalez-Garcia M, Caballero A, Jaramillo C, Torres-Duque CA.
Chronic bronchitis: High prevalence in never smokers and underdiagnosis— A
population- based study in Colombia. Chron Respir Dis [Internet]. 2019; 16:
1479972318769771. AvailÂable at: https://doi.org/10.1177/1479972318769771
19. Horner A, Soriano JB, Puhan MA, et al. Altitude and COPD
prevalence: analysis of the PREPOCOL PLATINO-BOLD-EPI-SCAN study. Respir Res.
2017; 18(1): 162.
20. Rojas YG, Duque CAT, Figueredo M del C, et al.
Estimación de la prevalencia de EPOC en Colombia a partir del Registro
Individual de Prestaciones de Servicios de Salud (RIPS). Rev Colomb Neumol
[Internet]. 2019; 31(1). https://revistas.asoÂneumocito.org/index.php/rcneumologia/article/view/325
21. Schiavi E, Stirbulov R, Hernández Vecino R, et al; Puma
Team. COPD screening in primary care in four Latin American countries:
methodology of the PUMA Study. Arch Bronconeumol. 2014; 50(11): 469-74.
22. Echazarreta AL, Arias SJ, Del Olmo R, et al; Grupo de estudio
EPOC.AR. Prevalence of COPD in 6 Urban Clusters in ArÂgentina: The EPOC.AR
Study. Arch Bronconeumol (Engl Ed). 2018; 54(5): 260-9.
23. López Varela MV, Montes de Oca M. Variabilidad en la
EPOC. Una visión a través del estudio PLATINO. Arch Bronconeumol
[Internet]. 2012; 48(4): 105-6. Available at:
http://www.archbronconeumol.org/es-variabilidad-epoc-una-vision-traves-articulo-
S030 0289611003309
24. Hinojosa F, C E. Enfermedad pulmonar obstructiva
crónica (EPOC). Acta Médica Peru [Internet]. October, 2009 [cited
on July 27 2020];26(4):188-91. Available at:
http://www.scielo.org.pe/scielo.php?script=sci_abstract&pid=S1728-591720090004000
01&lng=es&nrm=iso&tlng=es.