Autor : Samolski Daniel1
1 Organization of Direct Business Services (OSDE), Respiratory Medicine, Buenos Aires, Argentina
Correspondencia :Daniel Samolski - E-mail: dsamolski@gmail.com
Abstract
COVID-19
pneumonia generates both immediate damage due to the viral effects and distant
damage due to inflammatory immune deregulation. Systemic corticosteroid therapy
has proven to be beneficial in the first part of the process, but its
usefulness in post-acute damage is still unclear. The number of affected
patients makes it imperative to find a treatment that reduces potential
pulmonary sequelae. This series of cases included 18 patients admitted to
polyvalent private medical institutions of Buenos Aires City: 15 were male and
3 were female; age 58.4 ± 13.6 years. History of most common comorbidities: AHT
(4 patients), obesity (6 patients) and smoking (4 patients). Five patients had
no medical history. All patients showed dyspnea, oxygen desaturation, and
persistent or progressive tomoÂgraphic abnormalities 14 days after their
infection. All of them received dexamethasone according to current regulations.
Subsequently, given the poor evolution, they were administered oral and/or
intravenous corticosteroids with the same treatment used for secondary
organizing pneumonia (OP). A transbronchial biopsy was performed in 6 of the
patients, showing an OP pattern in 3 of them. Four weeks after the beginning of
the treatment, all of the patients showed clinical improvement expressed by
decreased dyspnea and the fact that they didn’t require oxygen anymore and that
all chest tomographies showed clearly reduced pulmonary parenchymal involveÂment.
Systemic corticosteroids administered in the post-acute period of COVID-19 have
a clinical and radiological beneficial effect.
Key
words: COVID-19
pneumonia, Secondary organizing pneumonia, Systemic corticosteroid therapy
Received: 6/30/2021
Accepted: 9/13/2021
Abbreviations
COVID-19
SARS-CoV-2
infection
NYHA
New
York Health Association
O2 oxygen
OP
organizing
pneumonia
CAT
computed
axial tomography
MRA
mechanical
respiratory assistance
FBC
fibrobronchoscopy
BAL
bronchoalveolar
lavage
TBB
transbronchial
biopsy
DAD
diffuse
alveolar damage
CIP
cell
interstitial pneumonia
mg/kg
milligrams
per kilogram of body weight
Introduction
Since
the beginning of the COVID-19 pandemic, there has been a lot of debate about
the usefulness of corticosteroids for the treatment. From the beginning, they
were thought to have even a potential harmful effect1;
subsequently they showed their usefulness in patients with severe acute
pneumonia with requirement of oxygen therapy or some type of ventilatory
support2. Patients who
overcome the acute phase of the disease may show clinical and radiological
alterations3-4-5 in the post-acute period, with
their long-term evolution not clearly known yet. It is very important to have
tested treatments in order to accelerate recovery and reduce potential sequelae6. Corticosteroids would
counteract the inflammatory process triggered by the viral infection and perpetuated
by an “uncontrolled” immune system7.
This case report tried to give at least an initial response to this hypothesis,
describing the clinical and radiological evolution of patients who received
that treatment.
Materials and Methods
For
this report, we included 18 patients with severe8
COVID-19 pneumonia who 14 days after the beÂginning of the
symptoms persisted with significant clinical alterations (dyspnea FC III – IV
according to the NYHA scale, not explained by any other cause), altered
oximetry readings (oxygen desaturation (O2)
breathing ambient air, not present before COVID-19) and/or tomographic
alterations (bilateral parenchymal infiltrates suggestive of organizing
pneumonia (OP) or late appearance of new infiltrates not explained by an infection
of a different etiology). The patients were evaluated and treated in 3
polyvalent private medical institutions of the Autonomous City of Buenos Aires.
All
the patients received treatment with dexamethasone during the acute period,
according to what was described in the Recovery study2,
indicating in some cases other therapeutic measures based on what was approved
at the moment of the hospitalization (convalescent plasma, hyperimmune equine
serum, hydroxychloroquine, antiretroviral medicines).
We
performed chest tomographies (CAT) upon hospitalization, in cases of clinical
changes showing worsening of the patient’s respiratory condition, at the
beginning of corticosteroid treatment and 4 weeks after the beginning of such
treatment. We used intravenous pulse corticosteroids (methylprednisoÂlone, 500
mg a day, 3 doses) in patients with mechanical respiratory assistance (MRA) or
spontaneous breathing with high O2 requirement through high flow
cannula or mask with reservoir bag. Patients with O2 requirement per conventional
nasal cannula or less than 5 liters/minute were prescribed oral corticosteroids
(meprednisone, 0.5 to 0.75 mg/kg/day). This regimen was administered after the
intraÂvenous dose in patients who required pulse dosing. The treatment was
extended for 3 to 6 months, like other organizing pneumonias9,
with progressive decrease depending on the clinical, oximetric and radiological
response. Patients with suspected aggregated infections underwent a
bronchoscopy (FBC) with bronchoalveolar lavage (BAL) and transbronchial
biopsies (TBB), provided that it was clinically possible and safe, in order to
dismiss the suspicion and also to try and record the anatomopathological
characteristics of the inflammatory process shown in the images.
Ethical consideration
This
draft is a series of case reports. Merely descriptive approaches focused on the
interpretation of the results have been adopted, trying to reach valid
conclusions. It hasn’t been produced in the context of a research trial with
control groups or randomized treatments. The patients signed their informed
consent upon hospitalization and before performing the bronchoscopy. It follows
the guidelines of the Personal Data Protection Act No. 25,236, particularly
sections 1, 5 subsection D, 8 and 11, subsection D.
Results
15
out of the 18 patients included were male and 3 female, with a mean age of 58.4
± 13.6 years. There were five patients without a pathological history. The
other 13 had clinical and oncological medical history (Table 1). All the
patients were administered dexamethasone, 6 mg/day intravenously or orally for
10 days, according to the Recovery study. Mean time from the onset of symptoms
until the beginning of corticosteroid, “non-dexamethasone” treatment was 28.1 ±
10 days. Given the severity of their clinical condition, 7 patients initially
received intravenous treatment with methylprednisolone (5 patients with
high-flow nasal cannula or mask with reservoir bag and 2 with MRA). Those
treated with oral corticosteroids received meprednisone, 50 ± 12 mg/day.
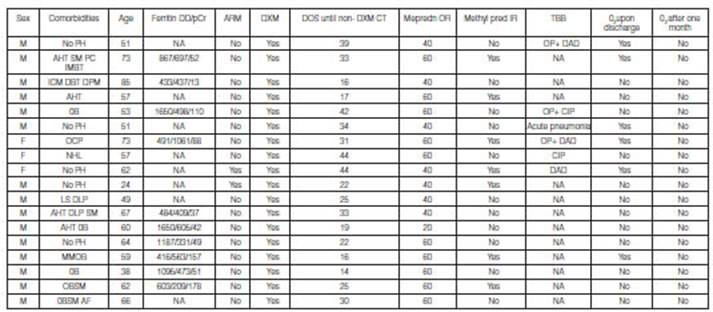
M: masculine. F: f
eminine. No PH: no pathological history. AHT: arterial hypertension. SM: sm oking . PC: prostate cancer. IMBT: inferior maxillary bone
tumor. ICM: ischemic cardiomyopathy. DBT: diabet es. DPM: definitive pacemaker.
Ob: obesity. OCP: ocular pemphigoid. NHL: non-Hodgkin lymphoma. LS: liver
steatosis. DLP: d ysli pidemia. M M : multiple
myeloma. AF: atrial fibrillation. Ferritin ng/ml. DD: D dimer ng/ml. pCR:
e-reactive protein mg/L. DOS: date of onset of symptoms. Non-DXM CT:
non-dexamethasone corticosteroid therapy. Mepredn OR: oral meprednisone,
initial dose. Methylpred IR: intravenous pulse methylprednisolone. TBB:
transbronchial biopsy. OP: organizing pneumonia. DAD: diffuse alveolar damage.
CIP: cell interstitial pneumonia. NA: not available/not performed. O2:
oxygen.
6
patients underwent FBC with BAL and TBB. No germs were isolated. The
anatomopathological report showed OP pattern in 3 patients: it was associated
with diffuse alveolar damage (DAD) in 2 cases, and with lymphocytic
inflammation or cell interstitial pneumonia (CIP) in 1 patient. One patient
showed isolated DAD changes, another patient had acute neutrophil inflammatory
damage and the remaining one reported CIP.
Six
of the 18 patients required home O2 supply
after hospital discharge, due to dyspnea or desaturaÂtion. One month after
discharge, none of the patients continued with the indication of O2 supply.
The
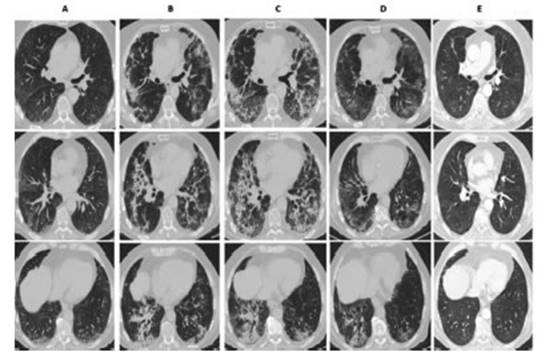
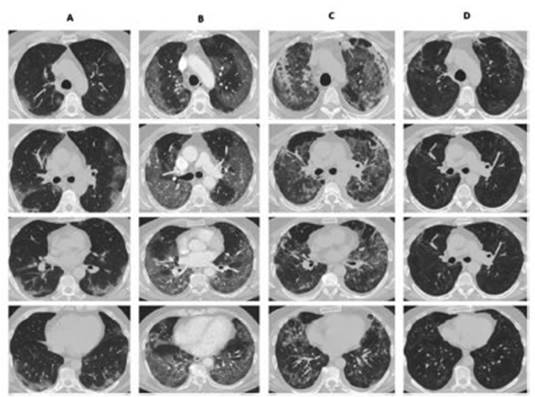
chest CAT performed 4 weeks after hospital discharge showed in all of the
patients a clear reÂduction of the parenchymal involvement, and the most
frequent finding was ground-glass persistence associated with septal thickening
(Figures 1 and 2). Only 1 patient had traction bronchiectasis and
another one showed images compatible with pneumatocele.
Discussion
When
the presence of the SARS COV 2 infection became known, it was assumed that the
clinical preÂsentation was the expression of the viral infection and the
post-acute period was the consequence of an immune system deregulation, more
commonly known as “cytokine storm”7. Corticosteroid therapy during
the infectious acute phase showed its usefulness in the Recovery study,2
which described an improvement in survival with the use of dexamethasone in
patients requiring some type of respiraÂtory assistance. Other authors reported
similar benefits using higher doses of methylprednisolone10-12. Once
the acute infectious process is resolved, like other more common etiologic
agents13, COVID-19 may evolve towards a clinical condition
compatible with secondary organizing pneumonia14-15. This is shown
in the tomographic characteristics observed during the evolution of the
infection6. In the anatomopathological necropsy reports and in some “in vivo”
biopsy reports, the damage pattern was confirmed, associated with other observed
patterns such as diffuse alveolar damage and acute fibrinous and organizing
pneumonia (AFOP)14, 17.
At
the moment, the natural evolution of the clinical and radiological consequences
post-acute COVID-19 infection is unknown. But given the number of patients
affected by this pandemic, it is imperative to find some treatment that
accelerates recovery and reduces respiratory abnormalities as potential
sequelae to the minimum. According to various reports3, 4, 18, 39%
of patients remained asymptomatic one month after hospital discharge, up to 63%
showed spirometric alterations 3 months after the infection and 30% after one
year, and 25% of the patients still showed radiological alterations one year
post-infection.
Myall
et al18 described in their work one approach that is similar to the one
described in this report, but they began corticosteroid therapy 6 weeks after
discharge in patients whose clinical or radiological findings were suggestive
of persistent pulmonary lesion, mainly OP. In that case they indicated only 3
weeks of treatment with oral corticosteroids and their results showed
symptomatic, radiological and spirometric improvement. The French guidelines19 for
the management of post-COVID respiratory seÂquelae also supported this
approach, considering all the patients who remained asymptomatic or with
radiological or spirometric alterations up to even 1 year post-infection as
capable of being treated. AcÂcording to these guidelines the treatment is more
similar to the conventional treatment of organizing pneumonia, starting with
prednisone, 0.5 mg/kg for one month and then reducing 10 mg every month.
Future
studies shall define if corticosteroid therapy has to be administered during
the immediate post-acute period or subsequently if there is no clinical or
radiological improvement. They must also evaluate whether there is a group of
patients that need the corticosteroid therapy of the acute period to continue
for more than 10 “formal” days in cases of radiological or clinical markers
suggestive of the subsequent “negative” evolution described herein. Finally, it
would be adequate to define the dose and duration of the corticosteroid therapy
considering that the underlying cause of the inflammatory process has been
resolved (acute viral infection), and which are the adverse effects related to
prolonged use.
This
retrospective work about a series of cases has clear limitations: the lack of a
control group and randomization of the indicated treatment. Treatment decision
making was defined by the attending physician, who clinically analyzed each
separate case. Still, the favorable results that were described allow us to
suggest that systemic corticosteroid therapy indicated after the acute period
would have a beneficial effect, both clinical and radiographic, in patients
with torpid evolution of severe pneumonia caused by COVID-19, provided that the
presence of pulmonary tromboembolism or bacterial or fungal superinfection or
other causes of dyspnea and/or pulmonary infiltrates (heart failure, drug-induced
pulmonary toxicity, exacerbation of underlying pulmonary diseases) that may
justify the clinical conÂdition beyond the unfavorable evolution of the
patient’s COVID-19 has been properly dismissed. It is necessary to conduct
prospective studies duly designed to clearly establish the benefit suggested in
this work, defining clear inclusion criteria, forms of therapy, dose and
duration.
Conclusion
Systemic
corticosteroid therapy administered after the acute period would have a
potential beneficial effect in patients with severe pneumonia caused by SARS
COV 2 who 14 days after the onset of sympÂtoms still show clinical or
radiological manifestations suggestive of damage generated by the immune
response to the virus.
Conflicts
of interest: The
author declares that there is no conflict of interest.
Acknowledgement:
To
the Internal Medicine Department of the IADT and Sanatorio Finochietto for
their participation and permanent support in the follow-up care of patients.
To
Dr.Teresa Castiglioni, pathologist of the Dr. Elsner
Laboratory, for her permanent cooperation with my work. And finally, I would
like to mention all the physicians and patients who confront this pandemic together.
References
1.
Centers for Disease Control and Prevention. Interim Clinical Guidance for
Management of Patients with Confirmed 2019 Novel Coronavirus (2019-nCoV)
Infection. Updated March 7, 2020.
2.
Horby P, WS Lim WS, Emberson J, et al. Dexamethasone in Hospitalized Patients
with Covid-19. The RECOVERY CollaboraÂtive Group. N Engl J Med 2021; 384:
693-704.
3.
Xiaojun W, Xiaofan L, Yilu Z, et al. 3 month, 6 month, 9 month and 12 month
respiratory outcomes in patients following COVID-19 related hospitalization: a
prospective study. Lancet Respir Med 2021. May 5:S2213-2600(21)00174-0
4.
Lerum TV, Aalokken TM, Bronstad E, et al. Dyspnoea, lung function and CT
findings 3 months after hospital admission for COVID-19. Eur Respir J 2021;
57(4): 2003448.
5.
Sibila O, Albacar N, Perea L, et al. Lung function sequelae in COVID-19
patients 3 months after hospital discharge. Arch Bronconeumol 2021; 57(S2):
45-63.
6.
Gentile F, Aimo A, Forfori, F et al. COVID-19 and risk of pulmonary fibrosis:
the importance of planning ahead. Eur J Prev Cardiol 2020; 27(13): 1442-6.
7.
Deblina Datta S, Talwar A, Lee JT. A Proposed framework and timeline of the
Spectrum of disease due to SARS COV 2 infection. Illness Beyond Acute infection
and Public health implications. JAMA 2020; 324(22): 2251-2.
8.
World Health Organization (2020) Clinical Management of COVID-19: interim
guidance; 27 May 2020. WHO
9.
King T. Cryptogenic organizing pneumonia. Retrieved May 13, 2021, from
www.uptodate.com/contents/COP
10.
Salton F, Confalonieri P, Meduri U, et al. Prolonged low doce methylprednisone
in patients with severe COVID-19 pneumonia. Open Forum Infect Dis. 2020; 7910:ofaa421. https://doi.org/10.1093/ofid/oaa421.
eCollection 2020 Oct.
11.
Edalatifard M, Akhtari M, Salehi, M et al. Intravenous methylprednisolone pulse
as a treatment for hospitalized seÂvere COVID-19 patients: results from a
randomized controlled clinical trial. Eur Respir J 2020;56:2002808
https://doi. org/10.1183/13993003.02808-202
12.
Papamanoli A, Yoo J, Grewal P, et al. High dose methylprednisolone in
nonintubated patients with severe COVID-19 pneuÂmonia. Eur Respir J 2020;
56(6): 2002808.
13.
Cordier JF. Cryptogenic organising pneumonia. Eur Respir J 2006; 28: 422-46.
14.
Edupunganti S, Kumar A, Konopka K. Organizing pneumonia as a manifestation of
coronavirus disease 2019. Pathol Int 2021; 7193: 210-2.
15.
Kory P, Kanne JP. SARS CoV2 organising pneumonia: Has there
been a widespread failure to identify and treat this prevalent condition in
COVID-19? BMJ Open Resp Res 2020; 7(1):e000724
16.
Parra Gordo ML, Buitrago Weiland G, Grau Garcia M, et al. Aspectos
radiológicos de la neumonía COVID-19: evolución y
complicaciones torácicas. Radiología 20212; 63: 74-88.
17.
Copin MC, Parmentier E, Duburcq T et al. Time to consider histologic pattern of
lung injury to treat critically ill patients with COVID-19 infection. Intensive
Care Med 2020; 46: 1124-6.
18.
Myall KJ, Mukherjee B, Castanheira AM, et al. Persistent Post COVID-19 Inflammatory
Interstitial Lung Disease: An obÂservational Study of corticosteroid treatment.
Ann Am Thorac Soc 2021; 18(5): 799-806.
19.
Andrejak C, Cottin V, Crestani ,B et al. Guide de
prise en charge des sequelles respiratoires post infection a SARS COV 2. Proposition
de prise en charge elaborees par la Societe de Pneumologie de Langue Francaise.
Versio du 10 novembre 2020. Revue des Maladies Respiratoires 2021; 38: 114-21.