Autor : FernĂĄndez Jesica Noelia1 Kevorkof Gregorio Varujan1 Acosta MarĂa Alejandra1 Castro Mara Soledad1 Oviedo Eduardo Enrique1 Najo Martin Augusto1 Ubal Leonardo GermĂĄn1 Yapur Bassani Natalia1 Lerda Marcelo1 Camporro Fernando1
1 Hospital TrĂĄnsito CĂĄceres de Allende, CĂłrdoba, Argentina
Correspondencia :
Abstract
There isnât yet a clear
definition for systemic inflammation in COPD (chronic obstructive pulmonary
disease), but its recognition has been based on studies that show an increase
in the plasma concentration of various inflammatory markers, such as the
c-reactive protein (CRP), and in recent years, also the microalbuminuria
has been suggested. The purposes of this work were to determine the microalbuminuria and CRP as potential biomarkers of
systemic inflammation. We enrolled patients with stable COPD and non-COPD
smokers diagnosed through spirometry; older than 40
years without AHT (arterial hypertension) or diabetes type I or II, between
October 2017 and March 2019. In both groups, a venous blood sample was
collected to determine high-sensitivity CRP and 3 urine samples were taken to
determine microalbuminuria, calculating the mean
value. At least two out of three determinations between 30 and 300 mg/g of
urine creatinine were considered to be significant
albuminuria. The high-sensitivity CRP was considered positive with a value ≥
5 mg/L. Of the 47 analyzed patients, a mean albuminuria of 13.91 ± 5.04 was
obtained in the COPD group, in comparison with 2.50 ± 0.36 in the control
group. Also, the high-sensitivity CRP mean values were compared, showing 5.06 ±
2.24 in COPD patients and 2.46 ± 0.51 in the control group. Both variables
showed non-statistically significant differences between the study groups (p =
0.058 for mean albuminuria and p = 0.330 for high-sensitivity CRP).
Key words:Microalbuminuria, CRP, Systemic inflammation, Biomarkers, COPD
Received: 12/12/2019
Accepted: 04/16/2021
Introduction
The inflammation pattern of
chronic obstructive pulmonary disease (COPD) includes neutrophils, macrophages
and lymphocytes (mainly CD8). These cells release inflammatory mediators which
attract cells from the blood flow that amplify the inflammatory process and
induce structural changes. That process is even more amplified by oxidative
stress and excess of proteases in the lung1.
Most patients with COPD have
chronic concomitant diseases related to the same risk factors: toÂbacco, aging
and inactivity, which cause a greater impact on prognosis and quality of life.
Inflammatory mediators on blood flow may trigger or worsen other diseases
present in these patients, such as: heart failure, ischemic heart disease,
arterial hypertension (AHT), osteoporosis, normocytic anemia, diabetes and
metabolic syndrome2.
However, there isnât yet a clear
definition of systemic inflammation in COPD. Its recognition has been based on
studies showing an increase in the plasma concentration of various inflammatory
markers: TNF<α (tumor necrosis factor <α, IL-6 (interleukin
6), IL-8 (interleukin 8), C-reactive protein (CRP), fibrinogen and leukocytes
in patients with stable COPD, compared to the same parameters in a normal
population3.
The release of these inflammatory
proteins hurts the endothelium, inducing a severe failure of blood microflow4. The vascular
endothelium, acting as a semi-permeable membrane, increases its permeÂability whenever
there is imbalance. Consequently, there is endothelial dysfunction that
facilitates an abnormal filtration of proteins5,
6.
In recent years, it has also been
suggested that microalbuminuria could be a predictor
of such endoÂthelial dysfunction. The glomerulus, as an extension of the
vascular endothelium, is also hurt during the systemic inflammatory response,
with severe consequences in local hemodynamic factors and the diameter of the
pores, thus resulting in protein filtration7.
Basing on prior history, microalbuminuria and high-sensitivity CRP could be
important indicators to predict systemic damage associated with COPD8.
Primary objective
To determine the microalbuminuria as potential biomarker of systemic
inflammation in patients with stable COPD compared to non-COPD smokers.
Secondary objective
To consider the high-sensitivity
C-reactive protein (CRP) as another possible indicator associated with this
disease.
Materials and methods
span class=GramE>Analytic,
observational, cross-sectional study. We used as a sample a group of patients who spontaneÂously went to the
outpatient offices of the Pulmonology Department of the Hospital TrĂĄnsito CĂĄceres de Allende and
signed the informed consent (version 1.3 â 2017 approved by the Institutional
Ethics Committee on Adult Health Research - Ministry of Health of the Province
of CĂłrdoba; attached as anÂnex), during the period between October 2017 and
March 2019.
Inclusion criteria:
1. Patients diagnosed with stable
COPD (defined according to GOLD [Global Initiative for Chronic Obstructive Lung
Disease] by a post-bronchodilator FEV1/FVC
[forced expiratory volume in first second/forced vital capacity] quotient <
0.70, confirming the presence of persistent airflow limitation in patients with
the appropriate symptoms and exposed to noxious stimuli, mainly tobacco smoke)9: stages 1 [mild], 2
[moderate], 3 [severe], and 4 [very severe] according to the GOLD
classification of COPD severity10,
previously diagnosed through spirometry.
2. Age > 40 years.
3. Males and females.
Exclusion< criteria:
1.
Arterial hypertension (AHT).
2. Diabetes mellitus 1 and 2
(DM).
3. Exacerbated COPD, defined as
acute worsening of respiratory symptoms that requires additional treatment;
increased dyspnea, cough and sputum volume and purulence, in the last year11.
4. Acute chronic kidney disease.
5. Urinary tract infection (UTI).
6. Gross hematuria.
7. Pregnant women.
8. Patients who had exercised and
had fever at the time of the consultation.
9. Pathological urine sediment.
Also, a control group was created
of male and female smokers, older than 40 years, without COPD (airflow
obstruction previously discarded by spirometry),
without AHT or DM type 1 or 2 or acute respiratory infection, plus the
remaining exclusion criteria previously established.
The following information was
confirmed from each patient: age, smoking load (packs/year); pathoÂlogical
personal history (according to medical records), and they all underwent a
thorough pulmonary physical examination including oxygen arterial saturation
and body mass index (BMI). Blood tests (see laboratory variables) and urine
samples were requested, taking into account the first morning urine of three
determinations with a maximum interval of one week between each sample. Patients
made 3 visits to the hospital: the first visit when they were enrolled, underwent the physical examination and blood
extraction and delivered the first urine sample; then the second and third
visits when they delivered the second and third urine samples, respectively.
Data obtained from each patient were registered in a form.
The following variables were
analyzed:
span class=GramE>o Demographic variables: age (years), gender, height (meters), weight
(kg).
o Clinical variables: systolic
arterial pressure (SAP) and diastolic arterial pressure (DAP) expressed in
mmHg, calculated with the sphygmomanometer technique recommended by the VIII
Joint ComÂmittee for AHT12,
BMI expressed in kg/m2, oxygen saturation by means of the portable Choicemmed MD300C pulse oximeter,
and pre- and post- bronchodilator spirometry,
performed with a Medgraphic spirometer according to
the recommendations of the ATS (American Thoracic Society)13,
using mainly the FEV1 (% of the value previously mentioned) as a spirometric variable.
span class=GramE>o Laboratory variables: complete cytological evaluation, high-sensitivity
CRP, glycemia, urea, creatiÂnine,
MDRD (Modification of Diet in Renal Disease) (glomerular filtration), complete
urine test and MAO (monoamine oxidase).
For determining the biochemical
variables, we collected 10 ml of venous blood from the ulnar or radial veins.
Serum samples were centrifuged at
200 rpm for 10 minutes in a Giumelli centrifuge and
processed in the COBA C 311 analyzer for determining the CRP (mg/L),
particle-enhanced immunoturbidimetric test.
For the complete urine tests, we
used SIEMENS Multistix reagent strips, Rolco 2080 centrifuge (1500 rpm, 5 minutes) and Labomed optical microscope (40x and 10x objectives). The
urine samples were centrifuged at 200 rpm for 3 minutes and then processed in
the COBA C 311 analyzer for the creatininuria and
albuminuria dosage, the latter by immunoturbidimetric
assay.
A patient was considered to have
significant albuminuria when at least two to three determinations had urine creatinine values between 30 and 300 mg/g (< than 30
mg/g was considered normal). With regard to the high-sensitivity CRP, it was
considered positive with a value ≥ 5 mg/L14-17.
Statistical analysis
Quantitative results were
expressed as mean ± standard error comparing all possible combinations of pairs
of mean values by multivariate ANAVA.
Qualitative results were
expressed as numbers (percentage) and analyzed with the Chi-Square Test.
A significance level of p<
0.05 was established for all the cases. All the tests were performed with the InfoStat program, version 2018e.
Results
Results were obtained from the
sample consisting of 47 individuals, one group of stable COPD (n= 27) and
non-COPD smokers from the control group (n= 20).
COPD-associated conclusive and
inconclusive variables were taken into account and used for comÂparative
analysis with the corresponding control group. Taking into account the
inconclusive variables, as shown in Table 1, both groups were homogeneous
regarding gender, anthropometric characteristics, white blood cells count
(including absolute and relative values of eosinophils
and neutrophils), glycemia and oxygen saturation (%).
This information is particularly interesting: the analysis of the group of COPD
patients yielded a mean of 97.07 ± 0.18 in comparison with the control group,
with a value of 97.45 ± 0.15, not showing significant differences between the
groups.
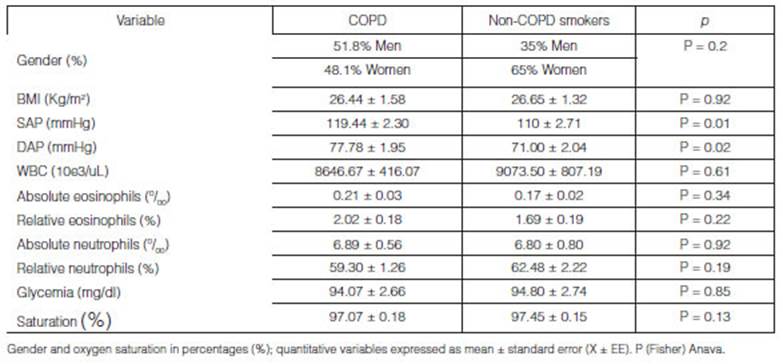
As regards the systolic and diastolic
arterial pressure, we found statistically significant differences (Table 1).
On the other hand, considering
the COPD-related conclusive variables, Table 2 shows age distribuÂtion,
smoking load (expressed in packs/year) and FEV1, with heterogeneity between the
groups.
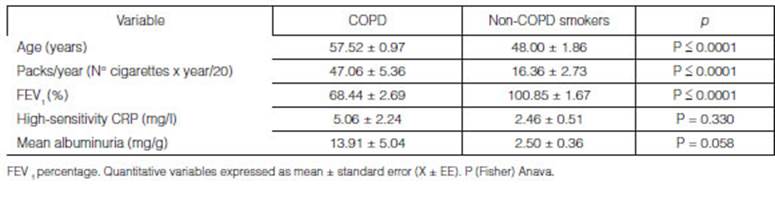
The degree of airflow obstruction
in patients with COPD is expressed in Figure 1. Most patients showed
mild and moderate obstruction, compared to a low percentage of severe and very
severe obÂstruction.
As regards the variables that are
the purpose of this study, we took into account the mean values for albuminuria
and high-sensitivity CRP of the COPD group (n= 27), in comparison with the
control group (n = 20) (Table 2). As for the albuminuria, since there
were 3 determinations corresponding to 3 different urine samples, we calculated
a mean value for those determinations that was analyzed together with the
high-sensitivity CRP.
Thus, a mean albuminuria of 13.91
± 5.04 was obtained in the COPD group, compared to 2.50 ± 0.36 in the control
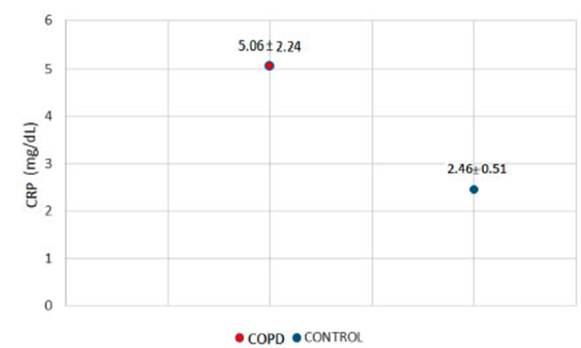
group (Figure 2). Also, the mean values for high-sensitivity CRP were
compared, with a value of 5.06 ± 2.24 in patients with COPD and 2.46 ± 0.51 in
patients from the control group (Figure 3). Both variables showed
non-statistically significant differences between the study groups (p=0.058 for
mean albuminuria and p=0.330 for high-sensitivity CRP).
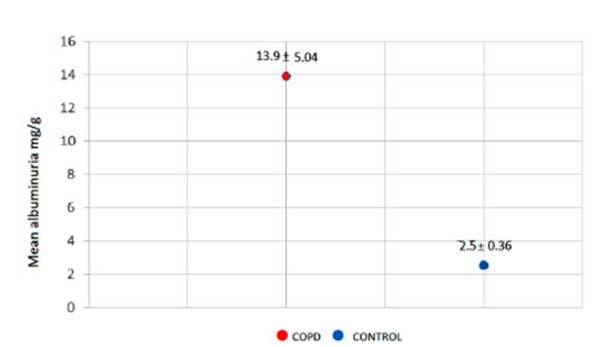
Discussion
The fact that COPD has systemic
effects in the form of structural and biochemical alterations in other organs
apart from the lungs is an emerging phenomenon. It has been reported that the
vascular endotheÂlium is an important site where the systemic effects of
inflammation occur; thus, the microalbuminuria is an
indirect manifestation of the systemic inflammation effect on the renal
endothelial permeability 18.
Various studies have suggested
the microalbuminuria as a biomarker of
COPD-associated systemic inflammation. Many of those studies considered not
only the patients with stable obstructive disease, and didnât discard other
diseases that cause microalbuminuria, such as AHT and
diabetes mellitus19-20-21.
It has been acknowledged that
COPD is frequently associated with a certain degree of systemic inflammation. So,
in patients undergoing stable stages of the disease, an increase in several
systemic inflammation markers in the peripheral blood has been described: TNF<α, IL-6, IL-8, C-reactive protein (CRP), fibrinogen, leukocytes and, in
recent years, microalbuminuria22.
In our area, it is one of the
first studies to determine a relationship of inflammatory markers, in this case
microalbuminuria and high-sensitivity CRP, between
patients with stable COPD and non-COPD smokers. A previous work from Casanova
et al showed a similar fact, suggesting that microalbuminuria
could help identify a subgroup of COPD patients with increased cardiovascular
risk and a potential adverse prognosis. In that study, COPD patients showed
significantly higher levels of microalbuminuria compared
to smokers without obstruction, as opposed to what could be observed in this
study, probably due to a small sample size and the cross-sectional nature of
the study.
As for the CRP, it showed a
tendency to increase in the COPD groups compared to control groups. Since the
CRP was not significant, our study couldnât show its positive association, just
like it happened with microalbuminuria. It is
important to underline that Casanova et al didnât take this biomarker into
account, considering that microalbuminuria is related
to cardiovascular events and death to a greater extent than the CRP. These
findings werenât proven by our research. Perhaps they showed a larger
albuminuria increase in their group of stable COPD patients by not discarding
subjects with elevated numbers of AHT; in fact, they showed that patients with
albuminuria had higher levels of systolic arteÂrial pressure. They could
determine that PO2 and systolic arterial pressure were significant predictors
of microalbuminuria levels. The microalbuminuria
levels were inversely proportional to the PO2, thus establishing a high
prevalence associated with hypoxemia, another finding not proven by our work.
The main results of our study
consist in showing a CRP tendency to increase (which was found to be moderately
above the cut-off point) and higher mean albuminuria (despite the fact that the
values were within normal limits, without a microalbuminuria
range) in COPD patients compared to the control groups, which could show a
tendency to a higher degree of inflammation in patients with obstructive
disease. We think the data werenât statistically significant due to the reduced
sample size.
Even though the potential of the
COPD biomarkers is promising, at present there isnât any truly difÂfering marker
that allows us to accurately predict the development and progression of the
disease, the onset of exacerbations, the response to a particular treatment or
the risk of mortality23. More studies are necessary in order to
determine the correlation of certain biomarkers with systemic inflammation in
COPD patients.
To conclude, the determination of
albuminuria didnât show significant differences between the groups. Even though
a higher degree was evidenced in COPD patients compared to the control group,
it wasnât within the microalbuminuria range, so it
canât be considered as a biomarker of systemic inflammation in patients with
stable COPD.
As for the high-sensitivity CRP,
a tendency to increase was evidenced in COPD patients compared to the control
group, but no significant difference was proven between the groups so as to
consider it as another useful biomarker to predict associated systemic
inflammation.
References
1.
Kumar V, Abbas A, Fausto N. Robbins
y Cotran. PatologĂa estructural y funcional. 7ma
edición. Barcelona (España): Elsevier Saunders; 2009
(cap. 15 El PulmĂłn) p: 723-4.
2.
Alvar AgustĂ MD, Claus Vogelmeier MD, Celli B MD, et al. Global Initiative for Chronic Obstructive Lung Disease. U.S National Library of Medicine, Bethesda, USA.
2018. 12-15, Chapter 1: definition and overview, pathology, pathogenesis and
pathophysiology.
3.
Casanova Macario C, de Torres Tajes JP, CĂłrdoba LanĂșs E. EPOC: inflamaciĂłn
bronquial y sistémica. Arch Bronconeumol.
2010; 46(Supl 4): 9-15
4.
Resendiz-HernĂĄndez J, Camarena A. Mecanismos
inmunolĂłgicos de la respuesta inflamatoria en el EPOC, NCT. 2010; 69(4): 210-7.
5.
Kumar V, Abbas A, Fausto N. Robbins
y Cotran. PatologĂa estructural y funcional. 7ma
edición. Barelona (España): Elsevier
Saunders; 2009. 519-520: Cap 11; Vasos sanguĂneos.
6.
BadimĂłn L, Martinez-gonzĂĄlez
J. DisfunciĂłn endotelial. Rev Esp
Cardiol Supl. 2006; 6:
21-30.
7.
Alegre J, Alles A, Angerosa
M. Implicancia de la Proteinuria en el DiagnĂłstico y Seguimiento de la
Enfermedad Renal CrĂłnica. Sociedad Argentina de NefrologĂa. Documento de
Consenso. 2015. 1-22
8. Ford E, Cunningham TJ, Mannino DM. Inflammatory markers and mortality among US
adults with obstructive lung funcÂtion. Respirology. 2015; 20(4): 587-3.
9. Alvar
A, Claus V, Celli B, et al. Global Initiative for Chronic Obstructive Lung
Disease. <2019;(4).Chapter 2: diagnosis and initial assessment.
10.
Casas A, Sansores R, Tokumoto A. Recomendaciones para
el diagnĂłstico y tratamiento de la enfermedad pulmonar obstrucÂtiva crĂłnica.
AsociaciĂłn Latinoamericana de TĂłrax. EdiciĂłn 1; 2011.CapĂtulo 2: Curso clĂnico,
diagnóstico, espirométrico y estratificación de la
gravedad. 12-18
11.
Alvar A MD, Claus V MD, Celli B MD, et al. Global Initiative for Chronic Obstructive Lung
Disease. U.S National
Library of Medicine, Bethesda, USA. 2018. 100-2, Chapter 5: Management
of exacerbations
12.
GĂłmez-LeĂłn Mandujano A, Morales LĂłpez S, Ălvarez DĂaz CJ. TĂ©cnica para una
correcta toma de la presiĂłn arterial en el paciente ambulatorio. 2016; 59:
49-55.
13.
GarcĂa-RĂo F, Calle M, Burgos F, Casan P, et al. EspirometrĂa.
Arch Bronconeumol. 2013;
499): 388-401.
14.
Alegre J, Alles A, Angerosa
M, et al. Implicancia de la Proteinuria en el DiagnĂłstico y Seguimiento de la
enfermedad renal crĂłnica. Sociedad Argentina de NefrologĂa. Documento de
consenso. 2016. 8-18.
15.
Calabia E. Medida de la funciĂłn renal. EvaluaciĂłn del
cociente microalbuminuria. Valor de la tira reactiva
y del examen del sedimento urinario. Indicaciones para solicitar ecografĂa
renal. NefrologĂa. 2004; 24 (Supl 6): 35-46.
16.
Figueroa Casas J, Schiavi E, Mazzei
J. Recomendaciones de la EPOC en la Argentina. Medicina (Buenos Aires). 2012;72 (Supl 1): 2-15.
17.
Gorosito M, SantamarĂa R, AlcĂĄzar R. Documento de la Sociedad Española de
NefrologĂa sobre las guĂas KDIGO para la evaluaciĂłn y el tratamiento de la
enfermedad renal crĂłnica. NefrologĂa 2014; 34 (Supl 3): 302-16.
18. Agrawal
A, Garg R, Sahu D, Kumar M.
Study the association of COPD with early endotelial
dysfunction and its impact on cardiovascular system by estimating urinary
albumin creatinine ratio. Lung India. Pubmed. 2017; 34: 138-43.
19.
Saldias F, DĂaz O, Dreyse
J, C et al. EtiologĂa y biomarcadores de inflamaciĂłn
sistĂ©mica en las exacerbaciones leves a modÂeradas de la enfermedad pulmonar
obstructiva crĂłnica. Rev Med Chile. 2012;
140: 10-8.
20. Jhon
M, Hussain S, Prayle A,
Simms R, Cockcroft JR, Bolton CE. Tarjet renal
damage: the microvascular associations of increased aortic
stiffness in patients with COPD. John et al. Respiratory
Research. 2013; 14: 31.
21. Kaysoydu
E, Arslan S, Yildiz G, Candan F. Factors Related to Microalbuminuria
in patients with Chronic Obstructive PulÂmonary Disease. Clin
Exp Med. 2014; 235):
749-55.
22.
Morales A, Dreyse J, Diaz
O, Saldias F, Carrasco F, Lisboa C. Marcadores de
inflamación sistémica en pacientes ex fumadores con enfermedad pulmonar
obstructiva crĂłnica en etapa estable. Rev med Chile. 2010; 138: 957-64.
23.
Izquierdo Alonso JL. Futuro de los marcadores biolĂłgicos en la EPOC. Arch Bronconeumol. 2017; 53(10):
541-2.