Autor Ruiz Vanesa Romina, Padilla-LĂłpez Marlene, Peralta Hugo Alberto, MartĂnez Bernardo Julio, Grande-Ratti MarĂa Florencia, Otero-Castro Victoria
Hospital Italiano de Buenos Aires. Argentina
Correspondencia : Vanesa Romina Ruiz. vanesa.ruiz@hospitalitaliano.org.ar
Abstract
Objective: To describe the characteristics of patients with influenza A subtype H3N2 requiring ventilatory support during the 2017 outbreak, as well as the evolution of the disease and clinical results.
Materials and Methods: Retrospective cohort. We included all patients admitted to the Emergency Department with confirmed diagnosis of H3N2 during June 2017, requiring invasive or noninvasive mechanical respiratory assistance, high-flow nasal cannula treatment or continuous airway pressure.
Results: 34 patients were included; 52.9% men, mean age 81 years (Standard Deviation [SD] 10). Main comorbidities of patients on admission were: 73.5% hypertension, 44.1% chronic obstructive pulmonary disease and 76.5% congestive heart failure. The mean Charlson Index score was 6 (SD 2), the APACHE II median (Acute Physiology and Chronic Health Evaluation II) was 17 (IQR 14-20) and the SOFA median (Sequential Organ Failure Assessment) on day 1 was 5 (IQR 3-7). On admission, 23 patients required noninvasive ventilation, 5 continuous positive airway pressure, 4 invasive mechanical ventilation and 2 high-flow nasal cannula therapy. The rate of noninvasive ventilation failure was 47.8% (95% CI [confidence interval] 26.8-69.4) and finally 38.2% of patients were intubated and mechanically ventilated. Hospital mortality was 52.9% (95% CI 35.1-70.2).
Conclusions: A high mortality rate was observed among elderly patients with comorbidities during the H3N2 outbreak. Most patients underwent a noninvasive ventilation trial on admission, however a high percentage failed. The initial condition could have been interpreted as acute chronic obstructive pulmonary disease or congestive heart failure.
Key words: Emergency Services; Human influenza; Influenza A virus; Respiration; Artificial; Noninvasive ventilation; Argentina.
Introduction
Influenza virus is a respiratory virus of great global relevance, responsible for pandemics and epidemics with high mortality and morbidity1. During an epidemic, approximately 5-15% of the world population are estimated to get infected, associated with roughly 3-5 million cases of severe disease and between 290-650,000 cases of death2. Patients with history of chronic medical conditions (pulmonary or cardiovascular diseases or diseases associated with immunosuppression) have 30 times higher risk of hospitalization and death3. Currently, there are three known types of influenza virus: A, B and C. The A type is the most virulent, and is divided in two more frequent subtypes that circulate among humans, the H1N1 and H3N24.
During the 2017 seasonal period, a strong predominance of subtype H3N2 was reported globally. This virus was responsible for an increase in seasonal mortality, especially in adults older than 65 years5-7.
However, there are few reports of cases requiring ventilatory support in relation to this strain5, 8, 9. Also, almost all the literature published in Argentina about influenza A and mechanical ventilation is dedicated to H1N110-13, and up to date there is not local evidence about the impact of the H3N2 seasonal outbreak.
Thus, our objective was to describe the characteristics of patients with influenza A subtype H3N2 requiring ventilatory support who consulted our hospital’s Emergency Department during the 2017 seasonal influenza outbreak, as well as their evolution and clinical results.
Materials and Methods
Study Design: Retrospective cohort.
Study Setting: The Adult Emergency Department (AED) of a high-complexity university hospital (third level). Every year, this hospital receives 2,950,000 consultations and has a hospitalization capacity of 750 beds, 200 of which are for critical care. During the 06/01/2017- 06/30/2017 period, there was a total of 3602 AED consultations, in the A area (shock room), the B area (general hospitalization) and the C area (medical offices), according to the decreasing level of complexity, with an average of 120 a day.
Population: Consecutive sample of every patient treated in the AED of our hospital who required some kind of ventilatory support (invasive or non-invasive ventilation) due to acute respiratory failure (ARF) with confirmed influenza A subtype H3N2 between 06/01/2017 and 06/30/2017 inclusive.
Inclusion Criteria: The study included every patient older than 18 years during the June 2017 period with diagnosis of influenza A subtype H3N2 confirmed through polymerase chain reaction technique and requiring invasive or non-invasive ventilator support due to ARF. Data were extracted from the electronic medical records (EMRs) and the electronic inter-consultation system of the respiratory care staff of the AED.
Exclusion Criteria: Patients requiring ventilatory support for a non-respiratory cause. Patients in which the polymerase chain reaction test was not performed or had negative results for the diagnosis of viral infection. Error in the determination of laboratory tests regarding the virologic results.
Procedures Followed: At the AED we define the ARF requiring ventilatory support basing on admission clinical signs: respiratory rate > 25 breaths/minute (r/min), oxygen saturation (SpO2) < 90% breathing ambient air and increase in the work of breathing (WOB) evidenced by dyspnea, retraction, use of breathing accessory muscles or diaphoresis despite starting conventional oxygen therapy > 6 liters per minute (l/min)14. We selected the kind of ventilatory support based on the type of ARF. In cases of hypercapnic ARF we used non-invasive ventilation (NIV); in hypoxemic ARF due to cardiogenic pulmonary edema we used continuous positive airway pressure (CPAP) or NIV15 and in cases of hypoxemic ARF due to pneumonia or for comfort in cases of limitation of therapeutic effort, we used high-flow nasal cannula therapy (HFNCT)16, 17. We consider NIV successful if the patient require escalation to another invasive or non-invasive method during the first 24 hours after starting NIV treatment; and NIV is considered as a failure if the patient requires such escalation. Ventilatory parameters were adjusted according to each patient in order to decrease the WOB and prevent asynchronies. We considered the use of invasive mechanical respiratory assistance (IMRA) on admission if the patient showed any contraindication for the use of non-invasive methods18, or within the first hour of using some non-invasive method if the patients developed: impaired consciousness, hypotension despite adequate resuscitation, respiratory frequencies > 35 r/m or no decrease in comparison with respiratory frequency on admission, increase or no decrease in the WOB evidenced by the use of respiratory muscles and the ventilatory mechanics, secretions that are difficult to manage, decrease in pH or no improvement of the acid-base state, SpO2 < 90% despite high FiO2 (fraction of inspired oxygen), PaO2 (partial pressure of oxygen)/ FiO2 < 15019. In cases of limitation of therapeutic effort, no invasive measures were taken. The diagnosis of congestive heart failure (CHF) was interpreted according to the clinical criterion of the doctor in charge of the patient, and registered by that term in the EMRs (Electronic Medical Records) or else according to diuretics requirement during hospitalization. The diagnosis of acute chronic obstructive pulmonary disease (COPD) was
made according to the clinical criterion of the doctor in charge basing on signs and symptoms the moment it was presented and registered by that term in the electronic medical record. The signs and symptoms of COPD exacerbation were: dyspnea, cough, tachypnea and increase in the amount or quality of secretions. Ventilators used to apply IMV or NIV were: SERVO-i® SERVO-s® Maquet Critical Care AB, Sweden; and the masks for NIV were: oronasal, PerformaTrak sizes M and L, or full face masks, Performax sizes S, L and XL Respironics®, Philips, USA. For the HFNCT we used the AirvoTM2 Fisher & Paykel Healthcare Limited system, and for the CPAP, the Whisper Flow® CPAP System Respironics Novametrix, LLC, USA.
Information was extracted by systematic review of the hospital’s EMRs done by AED researchers, trained for that purpose. The hospital has a unique data repository system that integrates information from all lab results together with clinical and administrative information. We registered clinical and demographic information (sex and age) from each patient, as well as the evolution of his/her condition and main comorbidities. Determination of the APACHE II score was based on lab results and clinical findings within the first 24 hours after the patient’s admission to the AED. The Charlson Comorbidity Index was used as summative score of the state of preexistent diseases. Nasopharyngeal smear was tested for influenza using the multiplex polymerase chain reaction molecular amplification method with analysis of amplification products through capillary electrophoresis in GENOMELAB GeXP (Beckman Coulter). Respiratory co-infection was defined as positive bacterial isolate in the patient’s endotracheal aspirate or sputum within three days after taking the respiratory virus panel20. All the patients received antiviral treatment with oseltamivir on admission, and empiric antibiotic treatment was administered to patients in critical care units, according to the recommendations of the guidelines of the Argentine Society of infectious Diseases (SADI)21. If the patient had echocardiographic evidence of heart failure with preserved ejection fraction and brain N-terminal pro-brain natriuretic peptide (NT-proBNP) of more than 400 pg/mL22, it was considered as diastolic cardiac dysfunction. The procalcitonine value was considered as positive when it exceeded 0.25 ng/mL.
This study has been approved by the local Institutional Ethics Committee and was conducted taking into account every consideration related to the protection of clinical investigation participants included in the Declaration of Helsinki and in accordance with the Guide for Health-Related Research involving Humans (Resolution 1480/11) of the Nation’s Ministry of Health. The study did not imply any kind of risk for the patient, and all the information obtained from the EMRs was used by researchers in strict confidentiality.
Statistical Analysis: To describe qualitative variables, we used absolute and relative frequencies, reported as proportions, with their respective 95% confidence intervals. To describe quantitative variables, we used the mean and standard deviation (SD) or median and interquartile range (IQR), according to their distribution, tested with the modified Shapiro Wilk test. The statistical program we used was software STATA version 14.
Results
A total of 34 patients were included in the study. The flow chart of patients with confirmed diagnosis of influenza A subtype H3N2 during the seasonal outbreak of 2017 can be seen in Figure 1.
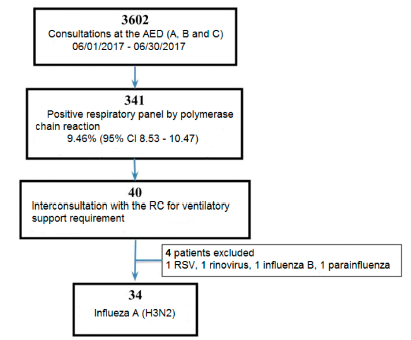
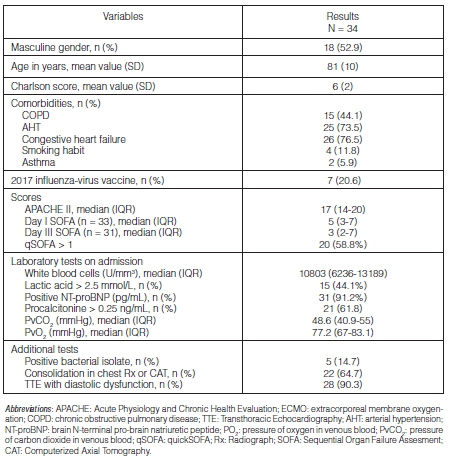
Most patients were male (52.94%), with a mean age of 81 years (SD 10). The mean Charlson score was 6 (SD 2). Regarding the history of the patients, 15 had COPD (44.1%), 25 were hypertensive (73.5%) and only 7 (20.1%) had received the influenza-virus vaccine in 2017.
On admission, 20 patients (58.8%) presented quickSOFA > 1. The APACHE II median at 24 hours was 17 (IQR 14-20). The SOFA was 5 on day I (IQR 3-7) and 3 on day III (IQR 2-7). Twenty one patients (61.8%) had procalcitonine > 0.25 ng/mL and 15 patients showed lactic acid > 2.5 mmol/L (44.1%). As positive findings, 28 patients (90.32%) showed a transthoracic echocardiogram (TTE) with diastolic cardiac dysfunction, the Pro-BNP was positive in 31 patients (91.2%), and 26 (76.5%) showed clinical ICC or required diuretics. We detected consolidation in chest x-ray or tomography in 22 patients (64.7%). Ninety one point two per cent of patients required short-lasting inhaled bronchodilators, and 29.4%
(10/34) required vasopressor support. The demographic characteristics, clinical variables, laboratory results and additional studies are shown in Table 1.
Most patients (23) used NIV on admission. Then, in order of frequency, CPAP (5), IMV (4) and HFNCT (2). The mean required CPAP levels were 8.75 cmH20 (SD 1.44) with mean FiO2 of 0.3 (SD 0.00), achieving a mean SpO2/FiO2 of 322 (SD 11). Also, the HFNCT requirements were: median of 50 L/min (IQR 40-60) and mean FiO2 of 0.38 (SD 0.08), achieving a mean SpO2/FiO2 of 266 (SD 50). The evolution of ventilatory requirements is shown in Figure 2.
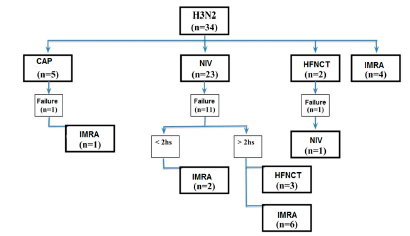
However, 41.2% (14) of patients needed to escalate to other type of ventilatory support, registering a NIV failure rate of 47.8% (95% CI 26.8-69.4). Table 2 shows the ventilatory settings of patients with NIV, differentiated between those who failed and those who succeeded.
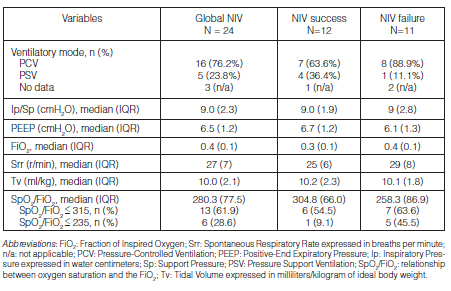
Finally, 13 patients received IMV (38.2%) with a median of duration of 4 days (IQR 2-7). Table 3 shows detailed ventilatory settings received on day 1, on day 3 and on the day of the patient’s death.
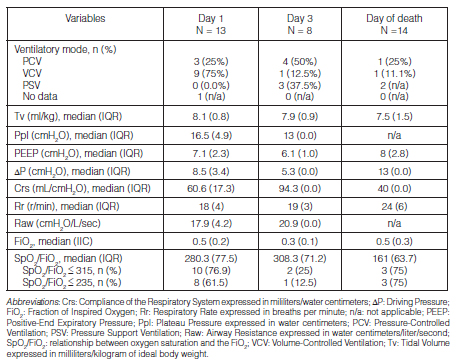
The median of AED length of stay was 2 days (IQR 1-3) and the mean length of hospital stay was 9.6 days (SD 6.2). Seven patients had to be referred to the Critical Care Unit (20.6% 95% CI 8.7-37.9). Global in-patient mortality was 18/34 (52.9% 95% CI 35.1-70.2), however, 14/34 (41.2%) patients had some degree of limitation of therapeutic effort.
Discussion
Most participants were elderly patients. Previous studies also observed a prevalence of H3N2 in patients older than 65 years23, 24. The vaccine is less effective in this age group, that is why it is the most affected by complications related to seasonal influenza3, 5. Also, our mean Charlson score exceeds 5 points, reflecting a death probability per year of more than 85%25.
Nearly all patients of our study required bronchodilators on admission. Airway resistance documented in patients receiving IMV was 4 times higher than the normal value. The influenza virus affects the respiratory epithelium from the nostrils to the bronchioles. The obstructive component associated with the infection in the airway might have had an important role on the onset of the ARF. We also observed a great number of patients with history of COPD, who have a more severe influenza infection, as has been previously described24.
Some authors explain that subtype H1N1 infects pneumocytes and intra-alveolar macrophages, causing greater alveolar dysfunction and, thus, greater development of acute respiratory distress syndrome (ARDS) and more severe conditions, even shock and multi organ failure10, 24. Due to the differences between subtypes H1N1 and H3N2 regarding physiopathology, clinical presentation, age group and severity, we think is not proper to make comparisons between the types of ventilatory support requirements used in our study and those with H1N110, 23, 26, 27. Even though most patients in our study were critically ill, proven by the high severity of the disease evidenced by the APACHE II median of 17, only 29.4% required vasopressor support. Also, our population doesn’t meet all the Berlin criteria28. Nearly all the patients had diastolic cardiac dysfunction evidenced by TTE. Since we didn’t have the PaO2/ FiO2 values on admission, we used the correspondence described by Rice et al for the SpO2/FiO229 values. Sixty one point nine per cent of patients receiving non-invasive ventilation had SpO2/FiO2 < 315 and only 28.6% showed < 235 values, so we didn’t consider it as a severe hypoxic respiratory failure.
According to current evidence on the use of NIV, it is not possible to make a recommendation in favor or against its use in cases of viral disease. Taking into account existing observational studies, most consider it reasonable to conduct a test with NIV in selected patients of experienced centers.15 Most patients underwent a NIV cycle at the beginning of the study. We understand it was related to the initial diagnosis of acute COPD or CHF, since the results of the viral panel by polymerase chain reaction took 5 days to be ready. We observed a high percentage of NIV failure. We think that the resistive component associated with airway inflammation due to the H3N2 infection was responsible for such failure. Also, the NIV applied to our patients may have increased the injury produced by mechanical ventilation by increasing tidal volumes exceeding 9 milimeters/kilogram (mL/kg) of ideal body weight.16 Probably the HFNCT could have been useful for reducing the resistive work without generating an injuring tidal volume (Tv), with more comfort for the patients.30 The two cases of bronchopneumonia by influenza A H3N2 reported by Doi et al were patients that met the ARDS criteria, who required IMV for less than 6 days and did not show bacterial, viral or fungal co-infection.9 The cases published by Collins et al and Shenoy et al describe an infection by H3N2 requiring IMRA in another age group, with ARDS, shock and co-infection5, 8. Unlike the published bibliography (57.6%), in our study there was low frequency of confirmed co-infection (14.7%)20. All the patients in our study received antibiotic treatment because they showed sepsis criteria: qSOFA > 1, positive procalcitonine and presence of consolidations in chest Rx or tomography.
Pneumonia mortality rates due to seasonal influenza are high, between 30 to 50%, depending on the existence or lack of ventilatory support and evolution to ARDS10. In our study, mortality was higher than 50%. However, we have to take into account the fact that it was an elderly, comorbid population, and a high percentage of patients had some degree of limitation of the therapeutic effort.
As health professionals of the emergency area, we are usually the first ones to treat patients with influenza. So, it is very important for us to have access to the most recent and relevant evidence when making decisions about the treatment of this disease4. Since there is little information about the type of ventilatory support for patients with H3N2, we believe our study has several strengths. First, it addresses the management of patients with ARF caused by influenza H3N2 at the emergency department, an area of knowledge where there’s still room for improvement. On the other hand, it is a homogeneous cohort of patients regarding age group, comorbidities, influenza virus subtype and ventilatory support requirements. Lastly, we provide data about the ventilatory setting used in each case.
Limitations of this study include the retrospective analysis of clinical data of a small number of subjects treated in only one health center and during a short period of time, limiting the generalization of our findings. Another limitation was the lack of data about FiO2 or values of gases in arterial blood
before the beginning of the treatment. Finally, the Tv ml/kg with NIV were excessive and an injury associated with mechanical ventilation may have been implicated. However, we are working in the design of a prospective study in order to evaluate the efficacy of HFNCT in patients with viral pneumonia.
To conclude, this H3N2 seasonal outbreak showed a high mortality rate in a predominantly elderly and comorbid population. Most patients conducted a NIV trial at the beginning of the study, which showed a high failure rate. The reason may have been the initial interpretation of the condition as acute COPD or CHF.
Acknowledgement: Dr. Naldo Genoud, Dr. Estela Salazar Schicchi and Dr. Diego Giunta for their advice. Dr. Myriam Peralta, Dr. Alberto Torrico and Lic. Ilona Bykhovsky for their cooperation in the presentation of the work at the International Conference on Emergency Medicine.
Conflicts of Interest: The authors of this study do not have any conflicts of interest to declare.
Funding: This study hasn’t been funded by any hospital entity or any other organization.
1. Ramos JM, García-Navarro MM, González de la Aleja MP, et al. Seasonal influenza in octogenarians and nonagenarians admitted to a general hospital: epidemiology, clinical presentation and prognostic factors. Rev Esp Quimioter. 2016; 29: 296-301. HYPERLINK “http://paperpile.com/b/vHQaHs/SzKz”
2. Gripe (estacional) [Internet]. World Health Organization. [cited 2018 Nov 18]. Available from: http://www.who.int/es/news-room/fact-sheets/detail/influenza-(seasonal)
3. Lee N, Choi KW, Chan PK, et al. Outcomes of adults hospitalised with severe influenza. Thorax. 2010; 65: 510-5.
4. Abraham MK, Perkins J, Vilke GM, et al. Influenza in the Emergency Department: Vaccination, Diagnosis, and Treatment: Clinical Practice Paper Approved by American Academy of Emergency Medicine Clinical Guidelines Committee. J Emerg Med. 2016; 50: 536-42. HYPERLINK “http://paperpile.com/b/vHQaHs/wmHR”
5. Collins LF, Anderson BD, Gray GC. A Case of Influenza A (H3N2) Complicated by Community-Acquired Pneumonia and Death in a Young Healthy Adult during the 2013-2014 Season. Front Public Health. 2017; 5: 1. HYPERLINK “http://paperpile.com/b/vHQaHs/yjxnX”
6. Vestergaard LS, Nielsen J, Krause TG, et al. Excess all-cause and influenza-attributable mortality in Europe, December 2016 to February 2017. Euro Surveill [Internet]. 2017;22(14). Available from: http://dx.doi.org/10.2807/1560-7917.ES.2017.22.14.30506 HYPERLINK “http://paperpile.com/b/vHQaHs/mmzGg”
7. Yang J-R, Hsu S-Z, Kuo C-Y, et al. An epidemic surge of influenza A(H3N2) virus at the end of the 2016-2017 season in Taiwan with an increased viral genetic heterogeneity. J Clin Virol. 2018; 99-100: 15-21. HYPERLINK “http://paperpile.com/b/vHQaHs/zc1UZ”
8. Shenoy ES, Lai PS, Shepard J-AO, et al. Case records of the Massachusetts general hospital. Case 39-2015. A 22-Year-Old Man with Hypoxemia and Shock. N Engl J Med. 2015; 373: 2456-66. HYPERLINK “http://paperpile.com/b/vHQaHs/6aJnY”
9. Doi M, Masao DO, Takao S, et al. Two Cases of Severe Bronchopneumonia due to Influenza A (H3N2) Virus. Detection of Influenza Virus Gene Using Reverse Transcription Polymerase Chain Reaction. Intern Med. 2001; 40: 61-7. HYPERLINK “http://paperpile.com/b/vHQaHs/vp2vy”
10. Valentini R. Reflexiones desde la Terapia Intensiva luego de la pandemia de virus de influenza H1N1-2009. Rev Am Med Resp. 2009; 9: 140-8. HYPERLINK “http://paperpile.com/b/vHQaHs/IptKm”
11. Ríos FG, Estenssoro E, Villarejo F, et al. Lung function and organ dysfunctions in 178 patients requiring mechanical ventilation during the 2009 influenza A (H1N1) pandemic. Crit Care. 2011; 15: R201.
12. Raffo L. Epidemia de influenza a (h1n1) en la argentina experiencia del hospital nacional profesor alejandro posadas. Medicina (B Aires). 2009; 69: 393-423. HYPERLINK “http://paperpile.com/b/vHQaHs/5XrFd”
13. Aquino-Esperanza J, Rodriguez PO, Boughen S, et al. Enfermedad Respiratoria grave En Terapia Intensiva Durante La Pandemia por el virus de Influenza A (H1N1) 2009. Medicina. 2010; 70: 401-7.
14. Parke RL, McGuinness SP, Eccleston ML. A Preliminary Randomized Controlled Trial to Assess Effectiveness of Nasal High-Flow Oxygen in Intensive Care Patients. Respir Care. 2011; 56: 265-70. HYPERLINK “http://paperpile.com/b/vHQaHs/Q1cTi”
15. Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J [Internet]. 2017;50(2). Available from: http://dx.doi.org/10.1183/13993003.02426-2016
16. Frat J-P, Thille AW, Mercat A, et al. High-Flow Oxygen through Nasal Cannula in Acute Hypoxemic Respiratory Failure. N Engl J Med. 2015; 372: 2185-96.
17. Peters SG, Holets SR, Gay PC. High-flow nasal cannula therapy in do-not-intubate patients with hypoxemic respiratory distress. Respir Care. 2013; 58: 597-600. HYPERLINK “http://paperpile.com/b/vHQaHs/ehty2”
18. Joshi N, Estes MK, Shipley K, et al. Noninvasive Ventilation For Patients In Acute Respiratory Distress: An Update. Emerg Med Pract. 2017; 19: 1-20. HYPERLINK “http://paperpile.com/b/vHQaHs/yF1hv”
19. Rello J, Pérez M, Roca O, et al. High-flow nasal therapy in adults with severe acute respiratory infection: a cohort study in patients with 2009 influenza A/H1N1v. J Crit Care. 2012; 27: 434-9. HYPERLINK “http://paperpile.com/b/vHQaHs/YtIFA”
20. Crotty MP, Meyers S, Hampton N, et al. Epidemiology, Co-Infections, and Outcomes of Viral Pneumonia in Adults: An Observational Cohort Study. Medicine 2015; 94: e2332. HYPERLINK “http://paperpile.com/b/vHQaHs/namRE”
21. Di Libero M, Gañete MJ, López Furst A, Mykietiuk C, Nemirovsky C, Osuna C, Pensotti P, Scapellato G. Neumonía Adquirida De La Comunidad En Adultos. Recomendaciones Sobre Su Atención. Medicina. 2015;75:245-57. HYPERLINK “http://paperpile.com/b/vHQaHs/l790P”
22. Redfield MM. Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2016; 375: 1868-77. HYPERLINK “http://paperpile.com/b/vHQaHs/tGHDe”
23. Kusznierz G, Cociglio R, Beltramino JC, et al. [Monitoring of activity of influenza in Santa Fe, Argentina, 2005-2010]. Rev Chilena Infectol. 2014; 31: 131-8. HYPERLINK “http://paperpile.com/b/vHQaHs/DnJzZ”
24. Chaves SS, Aragon D, Bennett N, et al. Patients hospitalized with laboratory-confirmed influenza during the 2010-2011 influenza season: exploring disease severity by virus type and subtype. J Infect Dis. 2013; 208: 1305-14. HYPERLINK “http://paperpile.com/b/vHQaHs/GO5at”
25. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987; 40: 373-83.
26. Silvina Bustos Fernanda Bonet. Análisis descriptivo de los casos de gripe a (h1n1) notificados durante la pandemia de 2009 en la región sanitaria v de la provincia de buenos aires, argentina. Rev Argent Salud Pública. 2010; 1: 6-10. HYPERLINK “http://paperpile.com/b/vHQaHs/WCkly”
27. Kusznierz G, Uboldi A, Sosa G, et al. Clinical features of the hospitalized patients with 2009 pandemic influenza A (H1N1) in Santa Fe, Argentina. Influenza Other Respi Viruses. 2013; 7: 410-7.
28. ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012; 307: 2526-33.
29. Rice TW, Wheeler AP, Bernard GR, et al. Comparison of the Sp o 2 /F io 2 Ratio and the Pa o 2 /F io 2 Ratio in Patients With Acute Lung Injury or ARDS. Chest. 2007; 132: 410-7.
30. Kernick J, Magarey J. What is the evidence for the use of high flow nasal cannula oxygen in adult patients admitted to critical care units? A systematic review. Aust Crit Care. 2010; 23: 53-70. HYPERLINK “http://paperpile.com/b/vHQaHs/krTZe”