Autor : Marco SolÃs, Patricia Maggio, Raquel Quian, Mabel Candelino, Cristina RodrÃguez, Oscar Rizzo, Guillermo Menga
Respiratory Rehabilitation Hospital âMarÃa Ferrerâ
Correspondencia : Marco SolÃs E-mail: msolisaramayo@yahoo.com.ar
Abstract
Introduction: Asthma is a heterogeneous disease characterized by chronic infammation of the airways. It is characterized by certain respiratory symptoms such as wheezing,
dyspnea, chest tightness, and cough that varies over time and in intensity, also showing
variable airflow limitation. Asthma affects 1 to 18% of the world population.
Evidence suggests that inhaled corticosteroids may show early therapeutic effects (< 3 hours). This quick response would be linked to a topical effect (vessel constriction
of the airway mucosa) due to the potentiation of the adrenergic effect by modifying the
postsynaptic receptors.
Materials and Methods: A prospective, randomized, analytical and experimental
longitudinal cohort study was performed in patients with asthma attacks treated at the
emergency department of the Respiratory Rehabilitation Hospital “María Ferrer”.
Results: A total of 71 patients were evaluated over a period of 10 months. All the patients were admitted through the emergency department of the Respiratory Rehabilitation
Hospital “María Ferrer” after they had agreed to participate in the protocol by signing an
informed consent.
Both groups had 63% of significant responses (Forced expiratory volume in first second,
FEV1> 60%) 30 minutes after starting the treatment (p: 0.72). At the end of the protocol
(180 minutes), three patients of the control group (salbutamol + ipratropium bromide)
did not meet the FEV1 > 60% objective, compared to 2 patients of the group treated with
high-dose inhaled corticosteroids (p: 0.97).
Conclusion: Currently, the use of high-dose inhaled corticosteroids in asthma attacks
is a therapeutic option for patients with mild to severe attacks. The results of our study
do not provide significant evidence in support of the use of this medication to reduce the
incidence of hospitalizations or to significantly improve the pulmonary function.
Key words: Asthma exacerbation; Inhaled corticosteroid; FEV1.
Introduction
Asthma is a heterogeneous disease characterized
by chronic inflammation of the airways. It is characterized by certain respiratory symptoms such
as wheezing, dyspnea, chest tightness and cough
that varies over time and in intensity, also show-
ing variable airflow limitation. Asthma affects 1
to 18% of the world population.
The objectives of the treatment of a patient having an asthma attack are: a) Correcting hypoxemia
through oxygen administration (O2), b) Relieving airflow obstruction through repeated administration of inhaled bronchodilators and c) Reducing
inflammation and preventing relapses through the
administration of systemic corticosteroids2.
The evidence suggests that inhaled corticosteroids may show early therapeutic effects (< 3
hours)3. This quick response would be linked to
the non-genomic effect of corticosteroids due to the
potentiation of the adrenergic effect by modification of postsynaptic receptors4.
To achieve maximum efficacy, inhaled corticosteroid administration should be made repeatedly, together with beta-agonists. The work of Edmonds
et al concludes that inhaled corticosteroids reduce
hospitalizations, compared to placebo (OR [odds
ratio] = 0.30; CI [confidence interval] of 95%,
0.16-0.57), but their benefits are not clear when
compared to systemic corticosteroids5.
Our objective is to evaluate bronchodilator response by adding high-dose inhaled corticosteroids
to the usual salbutamol and ipratropium bromide
treatment, both groups being under treatment
with systemic corticosteroids.
Primary Objective
To compare the bronchodilator effect of salbutamol + ipratropium bromide in inhalation aerosol with salbutamol + ipratropium bromide + budesonide, all of them associated with systemic corticosteroids (IR bolus injection of dexamethasone 8 mg), and supplemental O2 (if the transcutaneous oxygen saturation is < 90%) in patients who attend the emergency department of our hospital with mild to severe asthma attacks.
Secondary Objectives
To evaluate, in both treatment arms:
1. The increase of glycemia.
2. The variation in blood potassium.
3. The hospitalization rate.
4. The recovery hours until hospital discharge.
Materials and Methods
A prospective, randomized, analytical and experimental, comparative study. Comparisons of
continuous variables were made with the t-test
or the Mann-Whitney U test. The Kaplan-Meier
method was used for time analysis, and the Log
Rank Test was used for comparing treatments over
time. The test results were considered significant
for an alpha level α ≤ 0.05.
All the statistical analyses were done with the
InfoStat statistical program, 2014 version. Faculty
of Agricultural Science, National University of
Córdoba. Some drawings were made with Excel
Microsoft Office 2007.
Asthmatic patients aged ≥ 18 years who came to the emergency department of the María Ferrer
hospital with mild to severe asthma attacks were
included in the study.
Asthma attacks were considered as mild to severe if they presented a FEV1 or peak espiratory
flow (PEF) of less than 60% of the theoretical
value, or ambient air O2 saturation of less than
95%, and/or clinical signs of respiratory failure6.
Patients were admitted in a numerical and consecutive way, the odd ones receiving the control
group treatment with salbutamol (S) + ipratropium bromide (IB) (treatment 1), and the even ones
receiving treatment with S + IB + budesonide
(Bud) (treatment 2). Both groups received systemic
corticosteroids at a dose of 8 mg of dexamethasone
by intravenous route.
A few clinical examinations were performed,
including the elements related to the current
exacerbation. The following data was recorded:
oxygen saturation, heart rate, spirometry with
FEV1 and peak-flow measurement, dyspnea level
measurement (modified Medical Research Council
[mMRC] scale), blood pressure and clinical evaluation of muscle retraction and wheezing.
We used: Medix OXI-3 pulse oximeter, series
number 1946, Vitalograph ALPHA III spirometer,
3-liter calibration syringe for spirometer with daily
spirometer calibration, bronchodilator treatment
including S + IB inhalation aerosol (100/21 mcg
per dose), Bud inhalation aerosol (200 mcg/dose)
and 8 mg ampoule of dexamethasone per intravenous route.
Oxygen was delivered with a nasal prong (if
O2 saturation was < 90%), and inhalation aerosols were applied with directly observed spacing
chamber.
Forced spirometry was performed before starting the bronchodilator treatment and every 30
minutes until reaching 3 follow-up hours, with
flow/volume curve printing and PEF measurement.
GROUP DESCRIPTION
Two groups:
1. Salbutamol + ipratropium bromide
2. Salbutamol + ipratropium bromide + budesonide
Salbutamol sulphate + ipratropium bromide in
one inhalation aerosol (100/21 mcg per dose) with
spacing chamber (making 7 respiratory cycles after
taking each puff), at a total dose of 400/84 mcg (1
puff at a time) every 20 minutes within the first
hour of treatment and then every 30 minutes until
completing 3 hours of treatment and/or reaching
60% of the FEV1 theoretical value.
Budesonide in inhalation aerosol (200 mcg per dose) with spacing chamber (making 7 respiratory cycles after taking each puff), at a total dose of 800 µgr (1 puff at a time) every 20 minutes within the first hour of treatment and then every 30 minutes until completing 3 hours of treatment and/or reaching 60% of the FEV1 theoretical value.
Inclusion Criteria
1. Patients with mild to severe asthma attacks
(worsening or acute exacerbation).
2. Patients shall sign the informed consent in order
to perform the study.
3. Patients shall agree to a physical examination,
spirometric evaluation and treatment.
Exclusion Criteria
1. Respiratory system disease (acute or chronic),
such as acute community-acquired pneumonia,
chronic obstructive pulmonary disease, pulmonary tuberculosis, cystic fibrosis, bronchiectases, diffuse interstitial pulmonary disease or
other disease other than asthma attack.
2. Lack of cooperation on the part of the patient
for the evaluation and treatment.
3. History of near-fatal asthma.
4. Smoking greater than or equal to 10 packs per
year.
5. Glycemia record of ≥ 200 mg/dL in the first venous blood sample.
6. History of chronic renal failure.
7. Patients with special needs or patients who
can´t read or write.
8. History of peripheral vascular disease that alters
pulse O2 saturation.
Results
The study was performed over a period of 10
months and evaluated a total of 71 patients. All
the patients were admitted through the emergency
department of the Respiratory Rehabilitation
Hospital “María Ferrer” and agreed to participate
in the protocol by signing an informed consent.
35 of the admitted patients also received inhaled
corticosteroids; 47.9% were male, 45% were admitted with level 2 dyspnea, according to the mMRC
scale, with a mean age of 43.7 years (+/-17.4) and
FEV1 mean values of 48.13% (+/-6.87) at admission. Both groups were comparable at the initial
evaluation.
No significant changes were observed between
the study groups, both of them being effective
treatments, where 93% of patients reached more
than 60% of the FEV1 margin of safety within the
first 3 follow-up hours (Table 1).
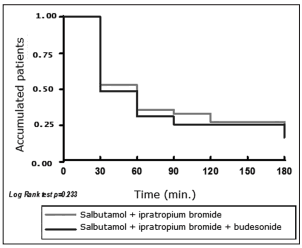
It was observed that 63% of the patients met
discharge criteria within the first 30 minutes,
with a mean improvement of 0.480 ml (+/- 0.333)
measured by FEV1, with no statistically significant
values between both groups (p: 0.72).
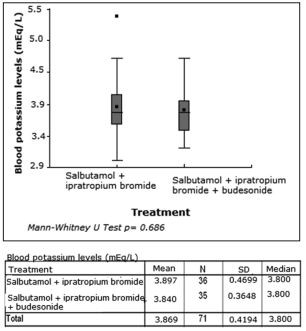
Variations in blood potassium levels (Table 2)
and pre- and post-treatment heart rate did not
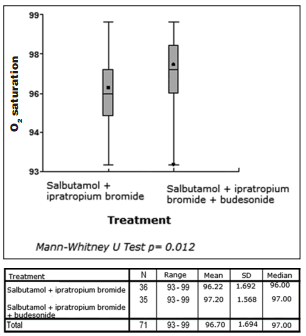
show significant changes, either. But, transcutaneous oxygen saturation showed significant differences between both groups after treatment, favoring the group treated with inhaled corticosteroids
(p: 0.012), with a mean saturation of 97% and a
FIO2 (fraction of inspired oxygen) of 0.21 (Table 3).
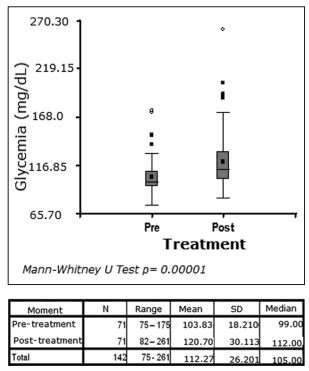
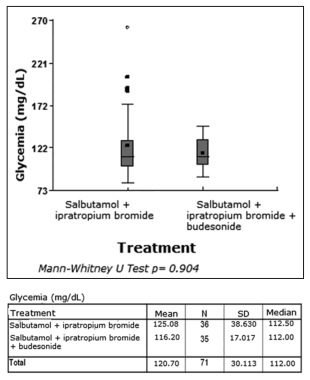
The difference between pre- and post-treatment
glycemia was also significantly representative,
with a p: 0.00001 value, but when both treatment
arms were subdivided, the result did not show any
statistically significant change favoring any of the
groups (p: 0.9) (Tables 4, 5).
Discussion
In physiopathological terms, bronchial asthma is a
disease that can be associated with inflammation
of the airways and an increase in the local bloodstream7. This increase in blood perfusion comes
from the bronchial arteries8 and is distributed to the subepithelial tissue. Inhaled corticosteroids are
the first line treatment for mild to severe asthma9.
Symptoms related to airflow limitation may be
reduced spontaneously or after taking medication, and even disappear for extended periods1. Despite
the efficacy and safety of their current treatment,
most patients are not thoroughly controlled.
Although some studies suggest a decrease in
their morbidity and mortality, many patients still
show exacerbations so severe that may put their
lives at risk and require immediate attention at
the emergency department7.
The guidelines of the Global Initiative for
Asthma (GINA) define an asthma attack as acute
or subacute worsening of the symptoms and pulmonary function of the patient with asthma that
modify his/her baseline condition and that in some
cases may be the first expression of the disease in
a patient with no previous diagnosis of asthma1.
Asthma attack is a frequent reason for consultation in the emergency department10. Approximately 15 to 25% of patients who come to the emergency
department may require hospitalization, and 10
to 20% of the patients who are discharged from
the emergency department will relapse within the
following 2 weeks11.
Rodrigo et al showed that the administration
of inhaled drugs is the method of choice for the
treatment of acute asthma, since it is associated
with a faster onset of action and less side effects,
as a consequence of the low doses that are required
to obtain the therapeutic effect at the airways and
the fact that some drugs are poorly absorbed into
the bloodstream2, 12, 13.
One of the most important factors for the efficacy of the bronchodilator treatment is the inhalation maneuver for drug administration and the
puff-inspiration coordination. There may be up
to 80% oropharyngeal deposition of the dose as a
consequence of a wrong inhalation maneuver. This
has been partially solved with the introduction of
one-way valve inhalation chambers placed between
the metered-dose pressurized inhaler (MDPI) and
the patient, reducing the oropharyngeal deposition
(10-15 times), with less side effects. The use of
these devices increases the amount of administered
drug that reaches the lungs, though not as much
as it reduces the oropharyngeal deposition (since
many particles are deposited in the walls of the
inhalation chamber), and reduces the treatment
time14.
In our study we used inhalation spacing chambers, since we could not obtain single-valve chambers. This way we make sure an effective dose
of the bronchodilator treatment is administered
and an adequate maneuver observed by experts is
performed, avoiding any mistake due to a wrong
management.
Currently there are very few studies that assess
the use of inhaled corticosteroids for the treatment
of asthma attacks. Marcia L et al made a metaanalysis about the use of inhaled corticosteroids
at the emergency department for asthma attacks.
The inclusion criteria for this meta-analysis
included studies comparing the use of inhaled
corticosteroids (IC) with placebo without using
systemic corticosteroids, and other studies comparing the use of IC with placebo plus the administration of systemic corticosteroids to both groups. 5
of the studies of the meta-analysis that reported
hospitalizations showed a significant reduction
of hospital admissions in the group treated with
inhaled corticosteroids, (OR [odds ratio] 0.30; CI
[confidence interval] of 95%, 0.16 to 0.57) with
no heterogeneity between the assessed studies,
but showed that 1 hospitalization was avoided
every 6 treated patients. The studies assessing the
pulmonary function showed a benefit in favor of
the use of inhaled corticosteroids, but without any
statistically significant values (p=0.15)15.
Edmond et al carried out a recent Cochrane
review showing the reduction in the hospitalization rate within the group of patients treated with
inhaled corticosteroids for asthma attack symptoms, but the studies assessed in this review use
patients who are compared to placebo or systemic
corticosteroids, none of them with fast-acting B2 agonists16.
Another evidence-based review was presented
by Rodrigo, showing a lower hospitalization rate,
a shorter length of stay at the emergency department and a very good FEV1 response in patients
who received high-dose inhaled corticosteroids
during the first hours of emergency care at the
emergency department; but it should be made
clear that most of the studies included in this review compare this effect with placebo or systemic
corticosteroids and not with the conventional,
standardized aerosol bronchodilator treatment17,
as in our study.
During our study, we did not observe a better
bronchodilator response in terms of the FEV1 or a lower hospitalization incidence within the
group of patients treated with high-dose inhaled
corticosteroids.
Both groups had 63% of significant responses
(FEV1 > 60%) 30 minutes after starting the treatment (p: 0.72). At the end of the protocol (180
minutes), the control group (S + IB) presented 3
patients who did not meet the FEV1 > 60% objective, compared to 2 patients of the group treated
with high-dose inhaled corticosteroids (p: 0.97).
Clinical and spirometric improvement in both
groups was remarkable within the first 60 minutes after starting the treatment. We believe one of
the most important factors influencing this quick
response was the administration of a controlled
treatment observed by experts with MDPI through
a spacing chamber. This suggests, for future studies, the possibility of obtaining a lower cost and a
greater benefit both for the asthmatic population
and for our hospital.
The hyperglycemia observed after administrating systemic glucocorticosteroids compels us to
provide extra monitoring of patients at risk.
Neither the blood potassium levels nor the
heart rate showed any significant changes after
treatment with fast-acting Beta 2 agonists. This
could be a consequence of the little exposure time
most of the patients had, since more than 50%
were discharged within the first 60 minutes after
starting the treatment.
Conclusion
Our study did not obtain statistically significant
values that support the addition of high-dose
inhaled corticosteroids to the conventional treatment in patients with asthma attacks.
An equally important issue was finding early
recovery in patients who received directly observed
treatment. This raises questions about the cost/effectiveness of the length of stay of these patients “under hospital roof”, in the emergency department.
We believe more studies are needed to assess
such response and evaluate its effectiveness at
reducing the hospitalization rate and also at being
objectively assessed in spirometric values.
Conflicts of Interest: The authors declare there is no conflict of interest related to this publication.
1. Global Initiative for Asthma. Global Strategy for Asthma
Management and Prevention. 2015 In:
http://www.ginasthma.org/guidelines-gina-reportglobal-strategy-for-asthma.html.
2. Rodrigo GJ, Rodrigo C. Tratamiento inhalatorio de las crisis asmática. Med Intensiva 2004; 28(2): 75-82.
3. Rodrigo G, Rodrigo C. Inhaled flunisolide for acute severe asthma. Am J Respir Crit Care Med 1998; 157: 698-703.
4. Kumar SD, Brieva JL, Danta I, Wanner A. Transient effect of inhaled fluticasone on airway mucosal blood flow in subjects with and without asthma. Am J Respir Crit Care Med 2000; 161: 918-21.
5. Edmonds ML, Camargo CA, Pollack CV, Rowe BH. The effectiveness of inhaled corticosteroids in the emergency department treatment of acute asthma: a meta-analysis. Ann Emerg Med 2002; 40: 145-54.
6. Rodrigo GJ, Plaza Moral V, Bardagí Forns S et al. Guía Alerta II. Arch Bronconeumol 2010; 46(7): 2-20.
7. Kumar SD, Emery MJ, Atkins ND, Danta I, Wanner A. Aireway mucosal blood flow in bronchial asthma. Am J Respir Crit Care Med 1998; 158: 153-156.
8. Deffebach ME, Charan NB, Lakshminarayanan S, Butler J. The bronchial circulation: small, but a vital attribute of the lung. Am Rev Respir Dis 1987; 135: 463-481.
9. Kumar SD, Brieva JL, Danta I, Wanner A. Transient effect of inhaled of Fluticasone on airway mucosal blood flow in subjects with and without asthma. Am J Respir Crit Care Med 2000; 161: 918-921.
10. Mannino DM, Homa DM, Pertowski C et al. Surveillance for asthma-United States 1960-1995. MMWR Morb Mortal Wkly Rep CDC Surveill Summ1998; 24: 1-27.
11. Camargo CA Jr, on behalf of the MARC investigators. Management of acute asthma in US emergency departments: the Multicentre Asthma Research Collaboration. Am J Respir Crit Care Med1998; 157: A623.
12. Rodrigo GJ, Rodrigo C. Aerosol and inhaled therapy in treatment of acute adult airway obstruction in the emergency department. Respir Care Clin N Am 2001; 7: 215-31.
13. Rodrigo GJ. Inhaled therapy for acute adult asthma. Curr Opin Allergy Clin Immunol 2003; 3: 169-75.
14. Van der Veen MJ, Van der Zee JS. Aerosol recovery from largevolume reservoir delivery systems is highly dependent on the static properties of the reservoir. Eur Respir J 1999; 13: 668-72.
15. Edmonds ML, Camargo Jr CA, Pollack CV, Rowe BH. The Effectiveness of Inhaled Corticosteroids in the Emergency Department Treatment of Acute Asthma: A Meta-Analysis. Ann Emerg Med 2002; 40: 145-154.
16. Edmonds ML, Milan SJ, Camargo Jr CA, Pollack CV, Rowe BH. Early use of inhaled corticosteroids in the emergency department treatment of acute asthma. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No.: CD002308.
17. Rodrigo GJ. Rapid Effects of Inhaled Corticosteroids in Acute Asthma an Evidence-Based Evaluation. Chest 2006; 130: 1301-1311.